NDC Code(s) : 71014-004-01, 71014-004-02
Packager : Eai-jr 286, Inc
Category : HUMAN PRESCRIPTION DRUG LABEL
DEA Schedule : none
Marketing Status : New Drug Application
INGREDIENTS AND APPEARANCE
| Foundation Sunscreen Face Broad Spectrum SPF 38 Titanium Dioxide STICK | ||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||
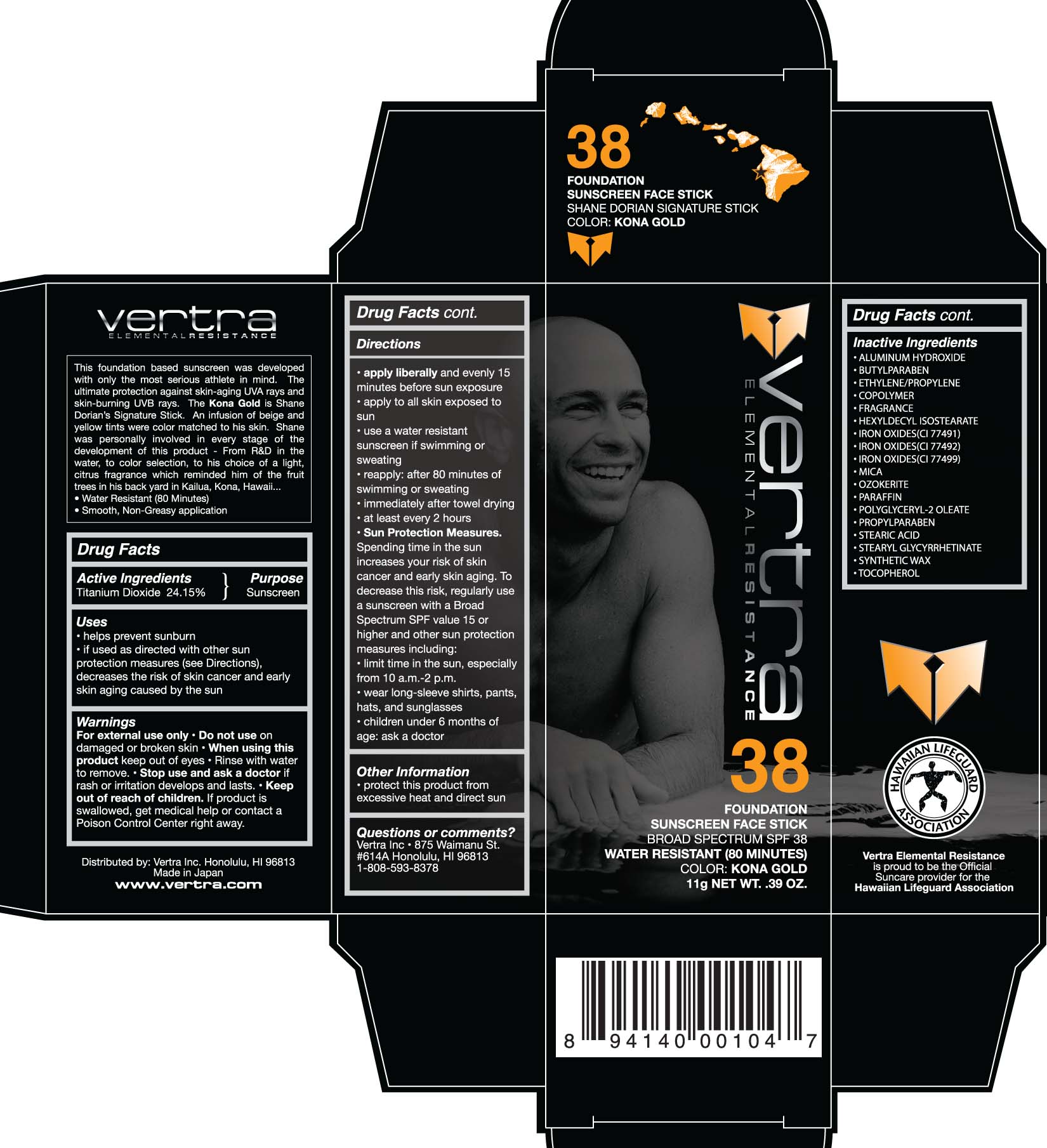
PRINCIPAL DISPLAY PANEL
Vertra Elemental Resistance
Foundation
Sunscreen Face Stick
Broad Spectrum SPF 38
Water Resistant (80 minutes)
Color Kona Gold
11g Net Wt. .39 oz