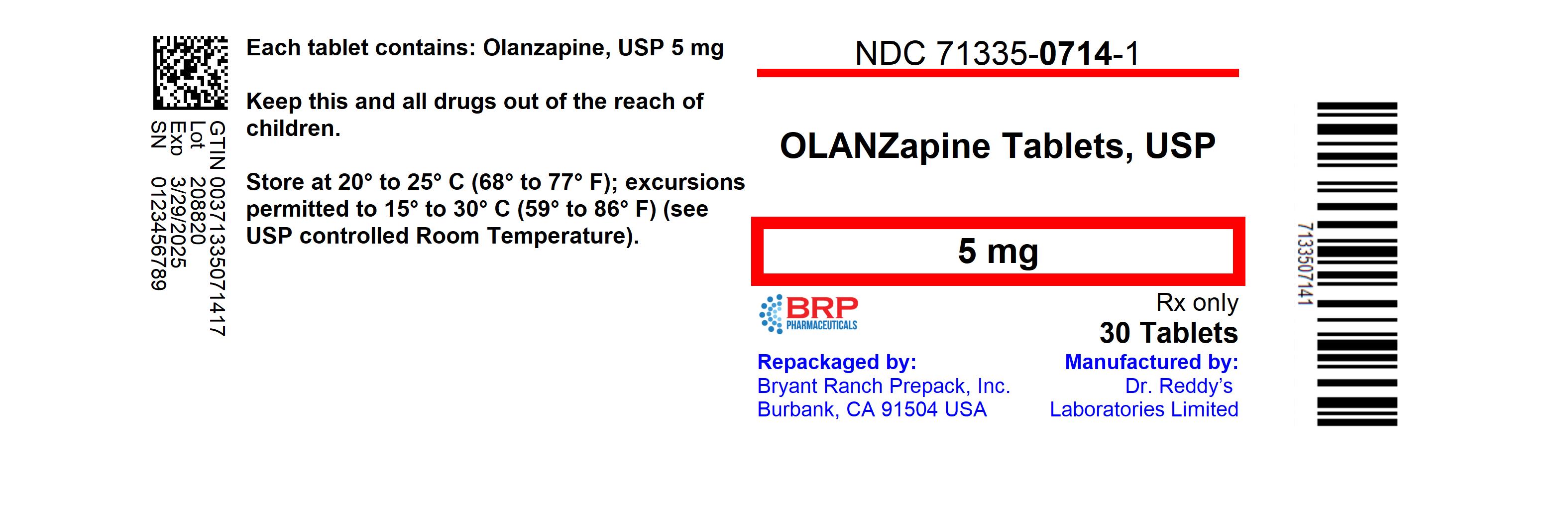
NDC Code(s) : 71335-0714-5, 71335-0714-1, 71335-0714-2, 71335-0714-3, 71335-0714-4
Packager : Bryant Ranch Prepack
Category : HUMAN PRESCRIPTION DRUG LABEL
DEA Schedule : none
Marketing Status : New Drug Application
INGREDIENTS AND APPEARANCE
| OlanzapineOlanzapine TABLET, FILM COATED | |||||||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||||||
| LABELER - Bryant Ranch Prepack(171714327) |
| REGISTRANT - Bryant Ranch Prepack(171714327) |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
| Bryant Ranch Prepack | 171714327 | REPACK(71335-0714), RELABEL(71335-0714) | |
PRINCIPAL DISPLAY PANEL
Olanzapine 5mg Tablet