NDC Code(s) : 71563-0400-1
Packager : Norris Ltd
Category : HUMAN OTC DRUG LABEL
DEA Schedule : none
Marketing Status : New Drug Application
INGREDIENTS AND APPEARANCE
| AB Skincare Acne Spot TreatmentBenzoyl Peroxide CREAM | ||||||||||||||||||||||||
|
||||||||||||||||||||||||
|
||||||||||||||||||||||||
|
||||||||||||||||||||||||
|
||||||||||||||||||||||||
|
||||||||||||||||||||||||
| LABELER - Norris Ltd(061314247) |
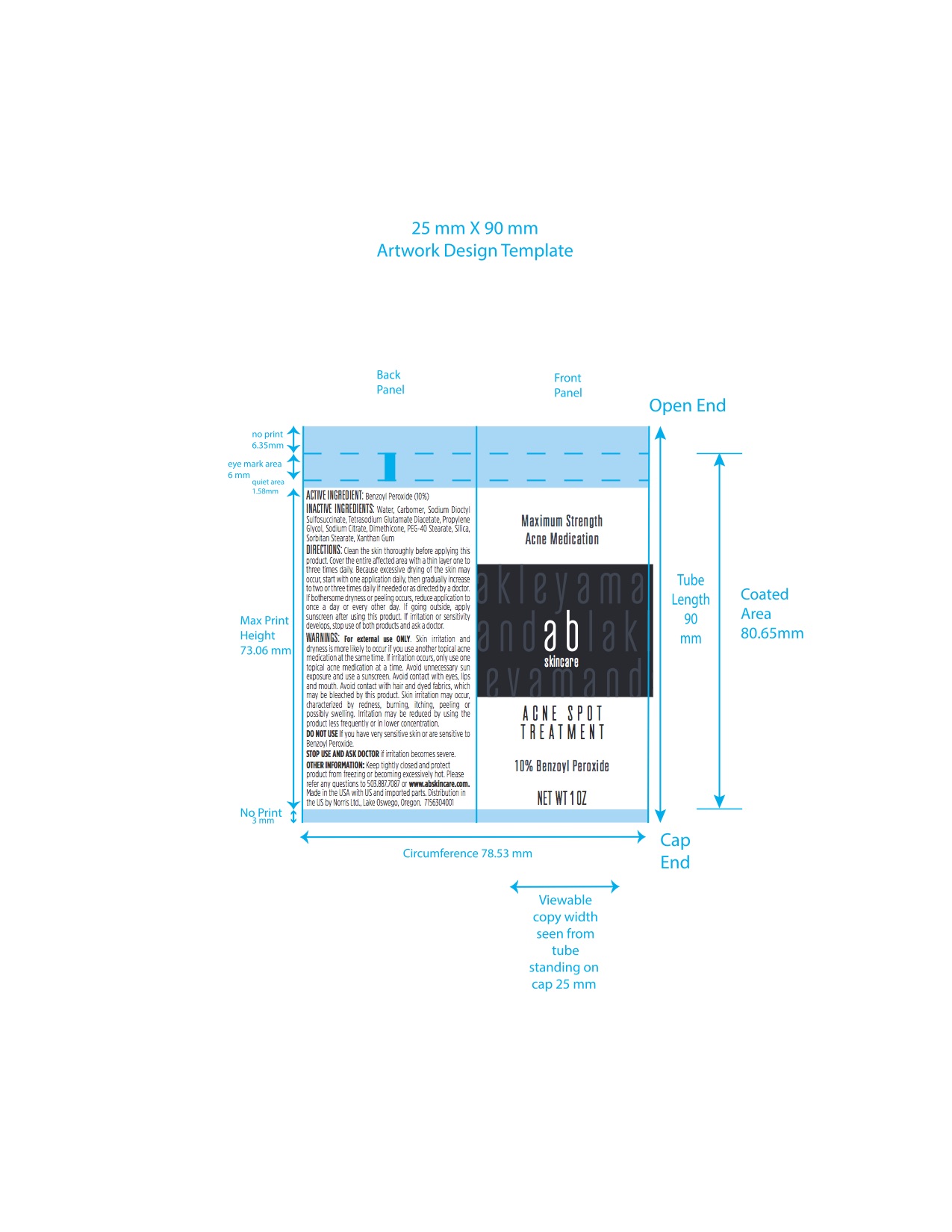
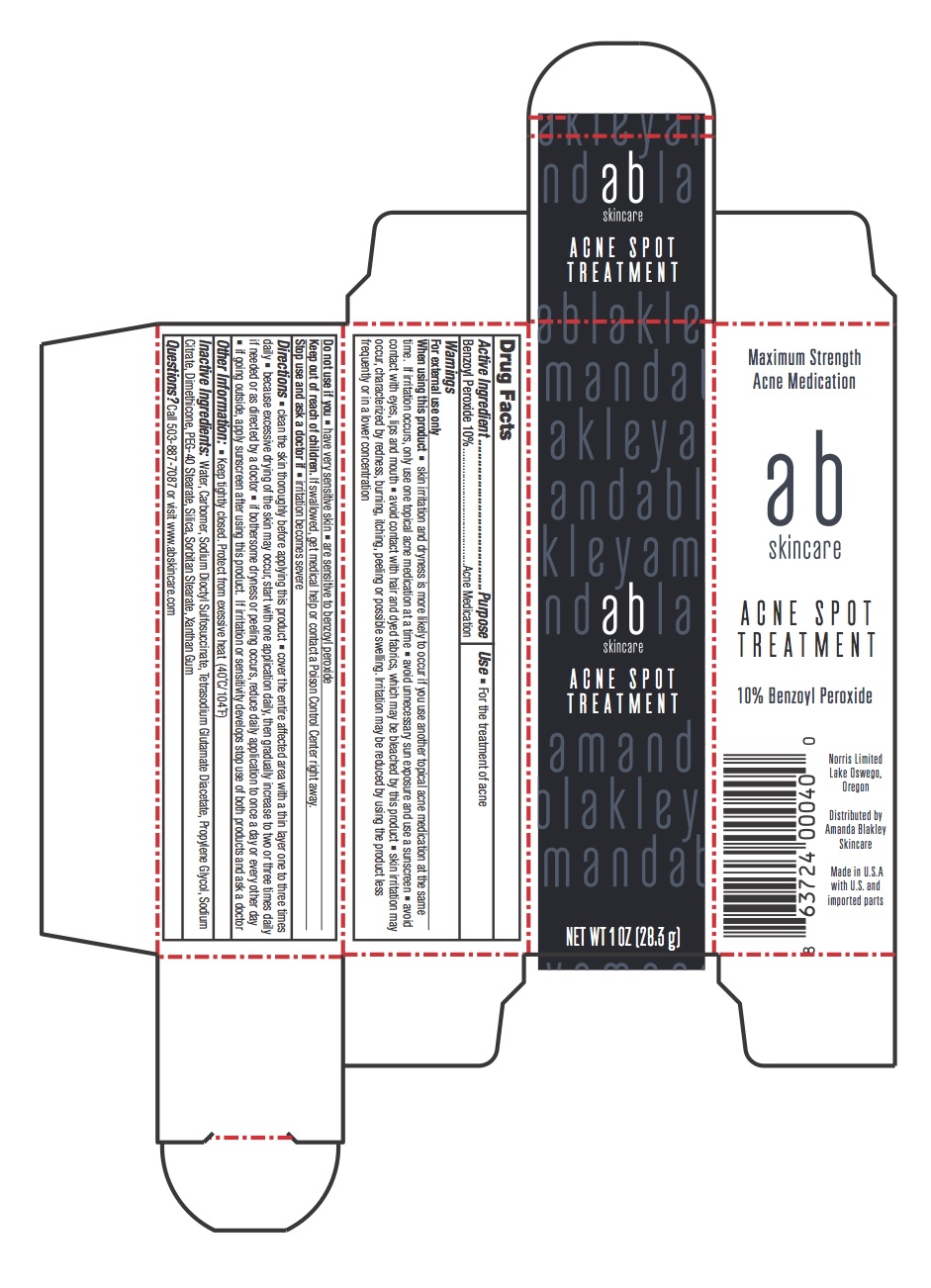
PRINCIPAL DISPLAY PANEL