NDC Code(s) : 71589-003-59, 71589-003-12
Packager : Aleor Dermaceuticals Limited
Category : HUMAN PRESCRIPTION DRUG LABEL
DEA Schedule : none
Marketing Status : New Drug Application
INGREDIENTS AND APPEARANCE
| Clobetasol Propionate Clobetasol Propionate SPRAY | ||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
| LABELER - Aleor Dermaceuticals Limited(871411532) |
| REGISTRANT - Aleor Dermaceuticals Limited(871411532) |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
| Contract Pharmaceuticals Limited Canada | 248761249 | MANUFACTURE(71589-003) | |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
| Aleor Dermaceuticals Limited | 871411532 | MANUFACTURE(71589-003), ANALYSIS(71589-003) | |
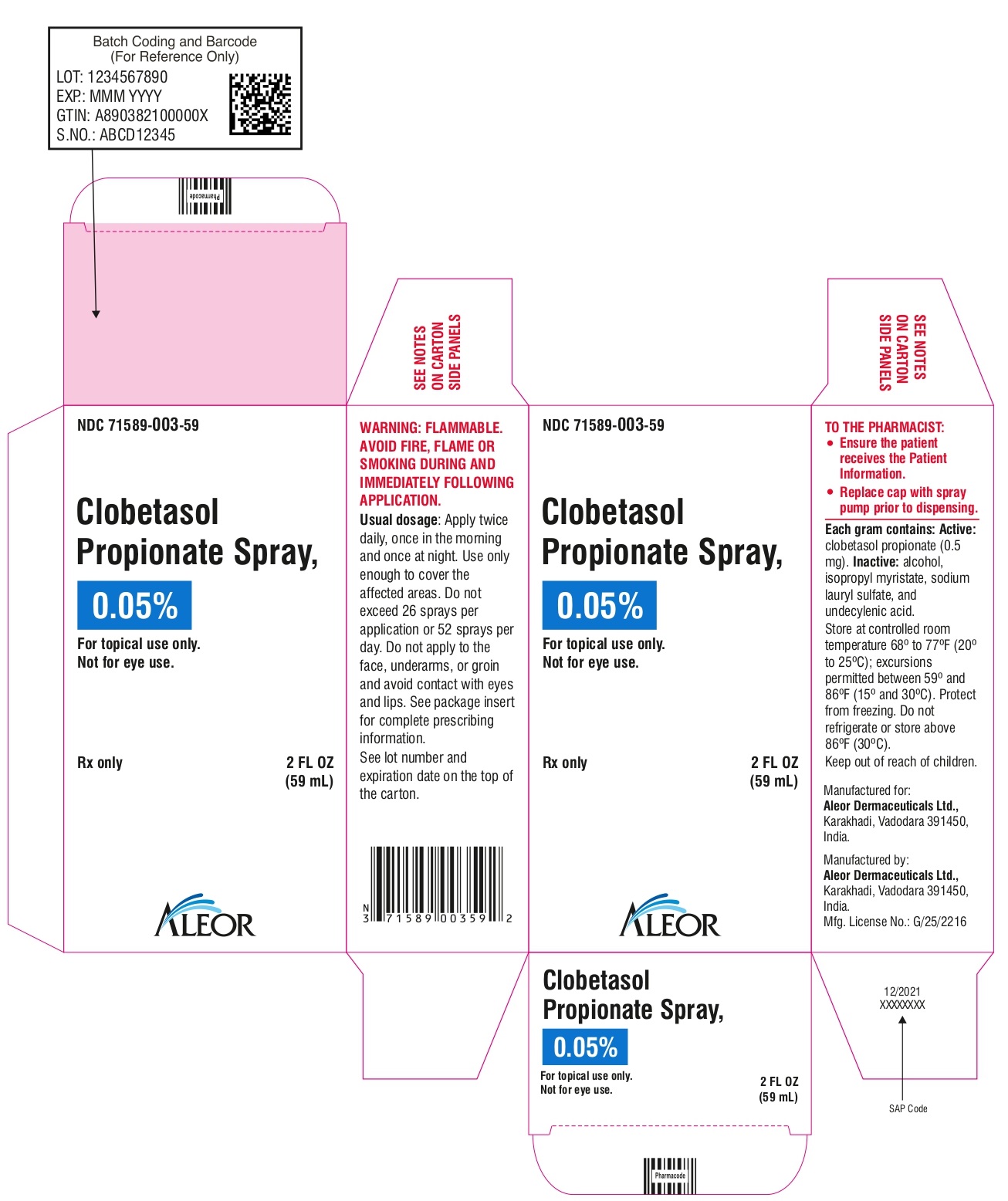
PRINCIPAL DISPLAY PANEL
Rx Only
NDC 71589-003-59
Clobetasol Propionate Spray, 0.05%
For topical use only
2 FL OZ
(59 mL)
Aleor Dermaceuticals Ltd.,
WARNING: FLAMMABLE AVOID FIRE, FLAME OR SMOKING DURING AND IMMEDIATELY FOLLOWING APPLICATION
Not for eye use.
Usual dosage: Apply twice daily, once in the morning and once at night.
Use only enough to cover the affected areas. Do not exceed 26 sprays per application or 52 sprays per day. Do not apply to face, underarms, or groin and avoid contact with eyes and lips. See package insert for complete prescribing information. See lot number and expiration date on the bottom of the carton.
TO THE PHARMACIST
- Ensure the patient receives the Patient Information.
- Replace cap with spray pump prior to dispensing .
Each gram contains: Active: clobetasol propionate (0.5 mg).
Inactive: alcohol, isopropyl myristate, sodium lauryl sulfate, and undecylenic acid.
Store at controlled room temperature 68° to 77°F (20° to 25°C); excursions permitted between 59° to 86°F (15° to 30°C).
Protect from freezing.
Do not refrigerate or store above 86°F (30°C).
Keep out of reach of children.
Manufactured for:
Aleor Dermaceuticals Ltd.,
Karakhadi, Vadodara 391450 India.