NDC Code(s) : 71589-007-04
Packager : Aleor Dermaceuticals Limited
Category : HUMAN PRESCRIPTION DRUG LABEL
DEA Schedule : none
Marketing Status : New Drug Application
INGREDIENTS AND APPEARANCE
| CLOBETASOL PROPIONATE CLOBETASOL PROPIONATE SHAMPOO | ||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
| LABELER - Aleor Dermaceuticals Limited(871411532) |
| REGISTRANT - Aleor Dermaceuticals Limited(871411532) |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
| Aleor Dermaceuticals Limited | 871411532 | MANUFACTURE(71589-007), ANALYSIS(71589-007) | |
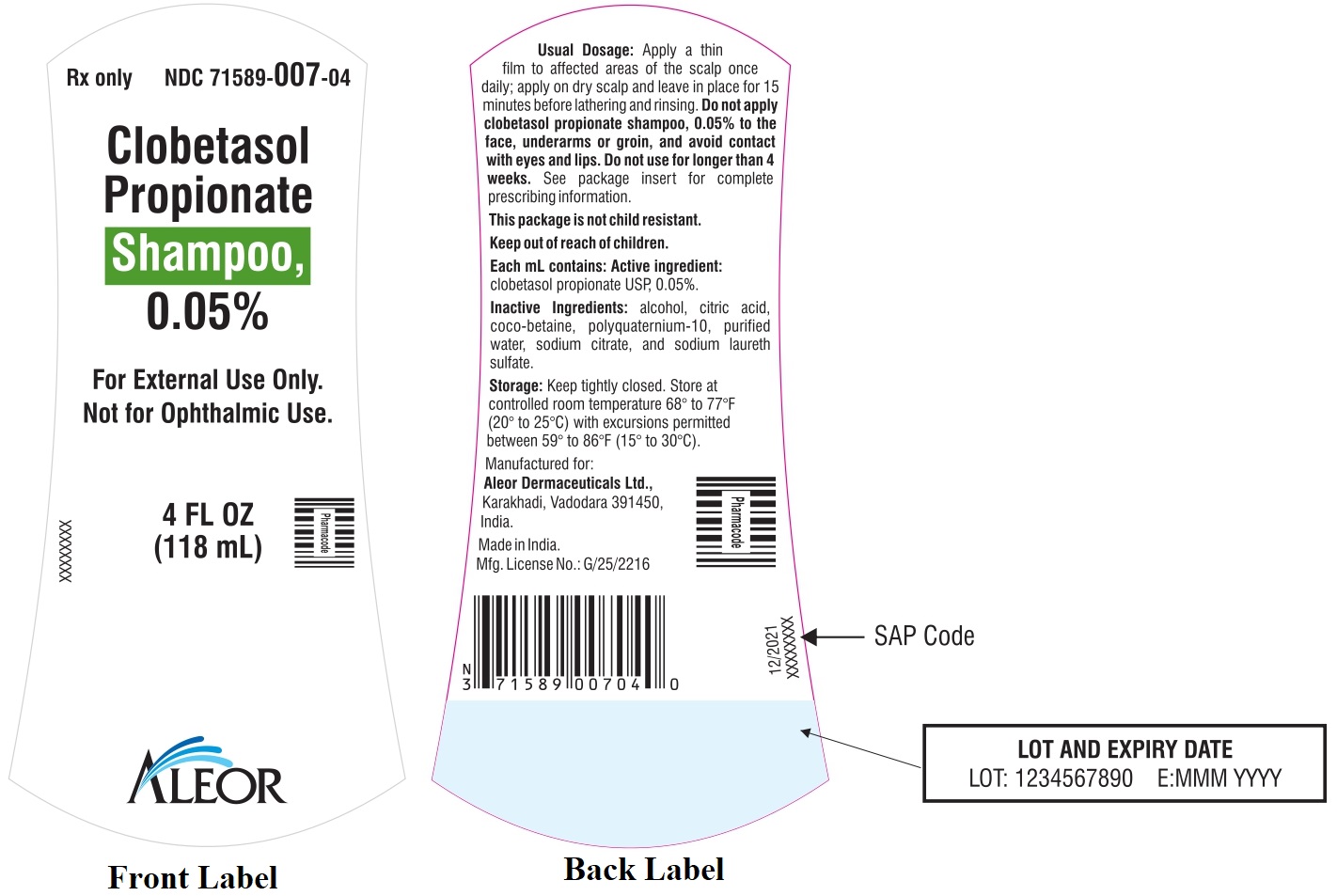
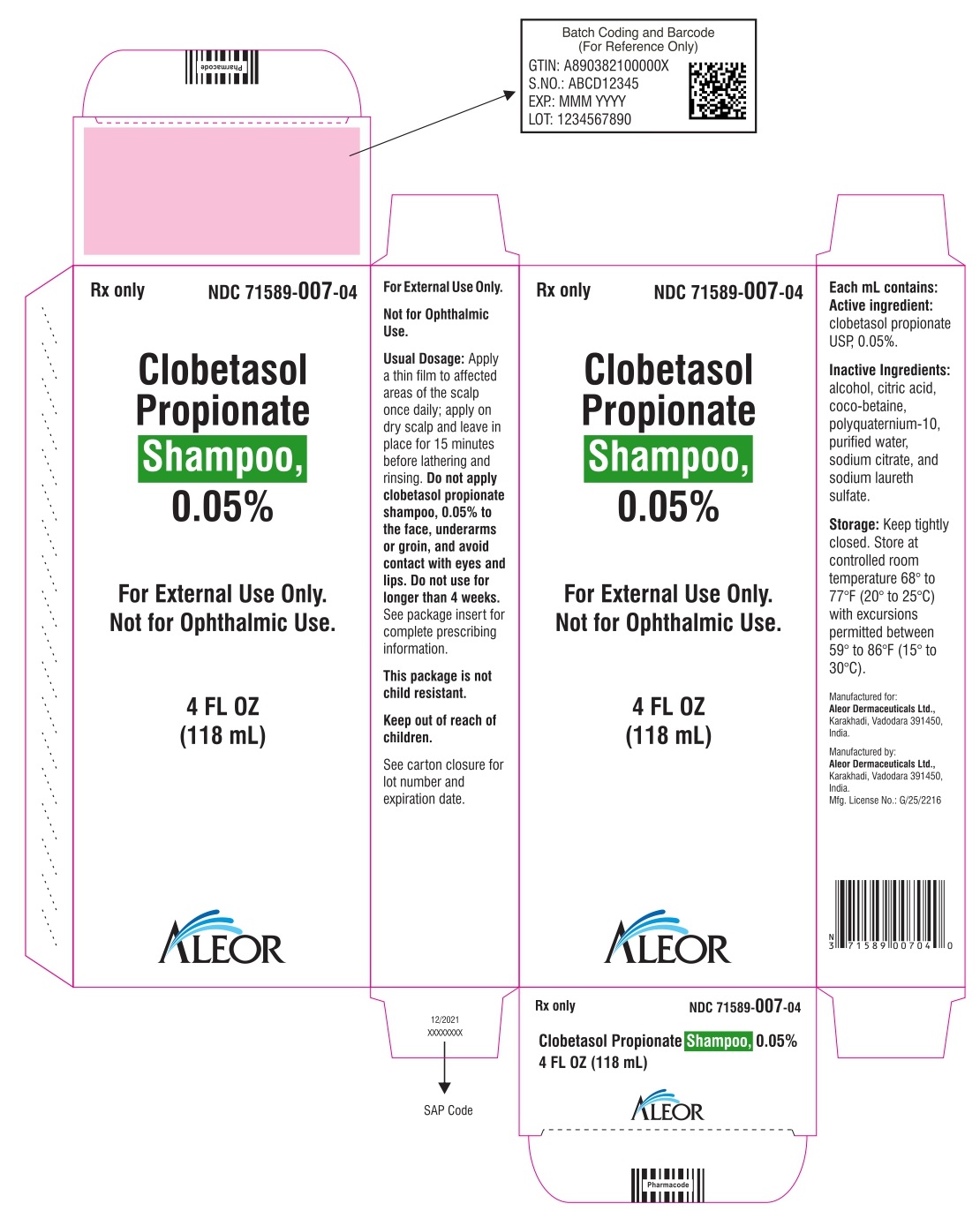
PRINCIPAL DISPLAY PANEL
Rx Only
NDC 71589-007-04
Clobetasol Propionate Shampoo, 0.05%
4 FL OZ
(118 mL)
ALEOR
For External Use Only.
Not for Ophthalmic Use.
Usual dosage:
Apply a thin film to affected areas of the scalp once daily; apply on dry scalp and leave in place for 15 minutes before lathering and rinsing. Do not apply clobetasol propionate Shampoo, 0.05% to the face, underarms or groin, and avoid contact with eyes and lips. Do not use for longer than 4 weeks. See package insert for complete prescribing information. See top of the carton for lot number and expiration date.
Each mL Contains: Active Ingredient: Clobetasol propionate USP, 0.05%.
Excipients: alcohol, citric acid, coco-betaine, polyquaternium-10, purified water, sodium citrate, and sodium laureth sulfate.
Storage: Keep tightly closed. Store at controlled room temperature 68° to 77°F (20° to 25°C) with excursions permitted between 59° to 86°F (15° to 30°C).
Manufactured by:
Aleor Dermaceuticals Ltd.,
Karakhadi, Vadodara 391450, India.
Mfg. License No: G/25/2216