NDC Code(s) : 71589-018-50, 71589-018-31
Packager : Aleor Dermaceuticals Limited
Category : HUMAN PRESCRIPTION DRUG LABEL
DEA Schedule : none
Marketing Status : New Drug Application
INGREDIENTS AND APPEARANCE
| CLOBETASOL PROPIONATE CLOBETASOL PROPIONATE AEROSOL, FOAM | ||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
| LABELER - Aleor Dermaceuticals Limited(871411532) |
| REGISTRANT - Aleor Dermaceuticals Limited(871411532) |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
| Aleor Dermaceuticals Limited | 871411532 | MANUFACTURE(71589-018), ANALYSIS(71589-018) | |
PRINCIPAL DISPLAY PANEL
NDC 71589-018-50
Clobetasol Propionate Foam, 0.05%
Rx only
50 grams
For Topical Use Only.
Not for Ophthalmic, Oral, or Intravaginal Use.
Invert can and then press firmly to dispense.
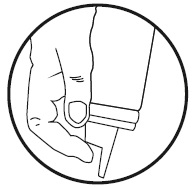
Description: Clobetasol Propionate Foam, 0.05%, contains 0.5 mg of clobetasol propionate, USP per gram in a thermolabile hydroethanolic foam vehicle consisting of cetyl alcohol, citric acid, ethanol (60%), polysorbate 60, potassium citrate, propylene glycol, purified water, and stearyl alcohol pressurized with a hydrocarbon (propane/butane) propellant.
Usual Dosage: Use only as prescribed by your physician. See accompanying prescribing information.
WARNING: FLAMMABLE. AVOID FIRE, FLAME, OR SMOKING











