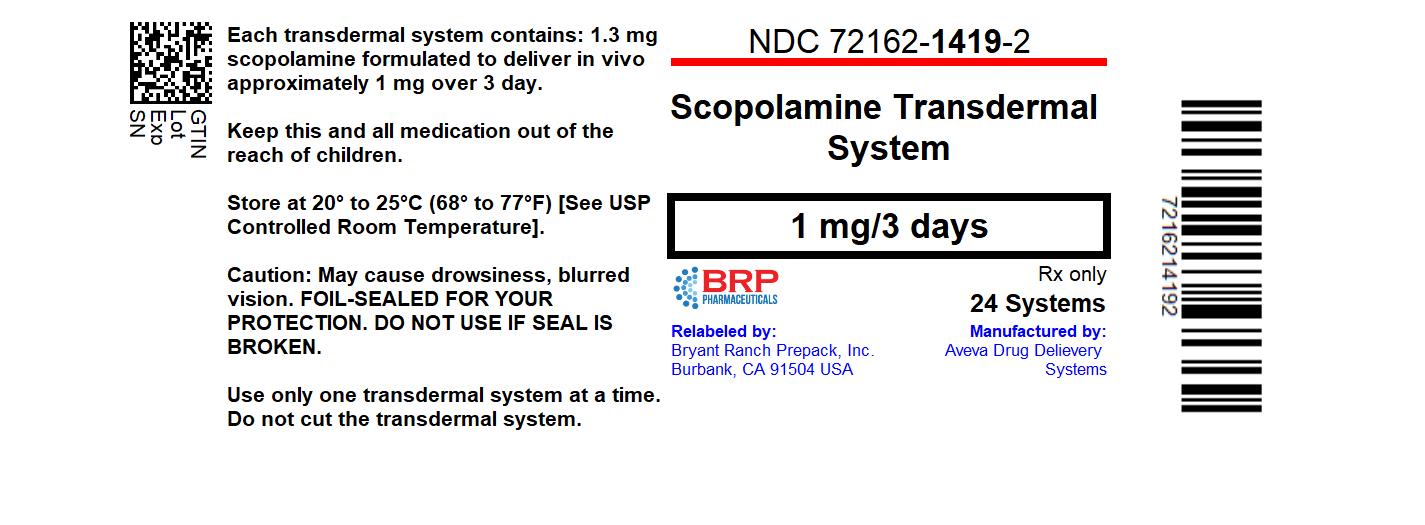
NDC Code(s) : 72162-1419-2
Packager : Bryant Ranch Prepack
Category : HUMAN PRESCRIPTION DRUG LABEL
DEA Schedule : none
Marketing Status : New Drug Application
INGREDIENTS AND APPEARANCE
| Scopolamine Trandermal Systemscolopamine transdermal system PATCH, EXTENDED RELEASE | ||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
| LABELER - Bryant Ranch Prepack(171714327) |
| REGISTRANT - Bryant Ranch Prepack(171714327) |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
| Bryant Ranch Prepack | 171714327 | REPACK(72162-1419), RELABEL(72162-1419) | |
PRINCIPAL DISPLAY PANEL
Scopolamine 1 mg Transdermal System, #24