NDC Code(s) : 72205-022-30, 72205-022-90, 72205-022-05, 72205-022-99, 72205-023-30, 72205-023-90, 72205-023-05, 72205-023-99, 72205-024-30, 72205-024-90, 72205-024-05, 72205-024-99, 72205-025-30, 72205-025-90, 72205-025-05, 72205-025-99
Packager : Novadoz Pharmaceuticals LLC
Category : Human Prescription Drug Label
DEA Schedule : none
Marketing Status : New Drug Application
INGREDIENTS AND APPEARANCE
| Atorvastatin calcium Atorvastatin calcium TABLET, FILM COATED | ||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
| Atorvastatin calcium Atorvastatin calcium TABLET, FILM COATED | ||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
| Atorvastatin calcium Atorvastatin calcium TABLET, FILM COATED | ||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
| Atorvastatin calcium Atorvastatin calcium TABLET, FILM COATED | ||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
| LABELER - Novadoz Pharmaceuticals LLC(081109687) |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
| MSN LABORATORIES PRIVATE LIMITED | 650786952 | ANALYSIS(72205-022, 72205-023, 72205-024, 72205-025), MANUFACTURE(72205-022, 72205-023, 72205-024, 72205-025) | |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
| MSN Pharmaceuticals Inc. | 079229051 | ANALYSIS(72205-022, 72205-023, 72205-024, 72205-025), MANUFACTURE(72205-022, 72205-023, 72205-024, 72205-025) | |
PRINCIPAL DISPLAY PANEL
10mg-90s-container-label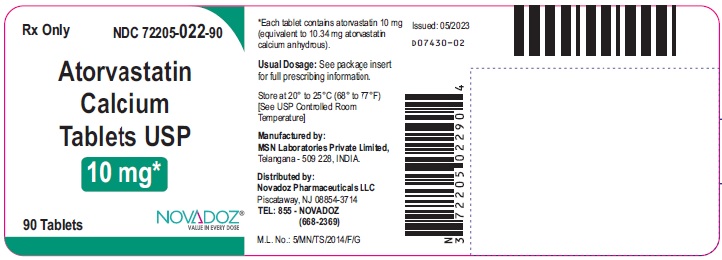
10mg-500s-container-label

10mg-1000s-container-label

20mg-90s-container-label

20mg-500s-container-label

20mg-1000s-container-label

40mg-90s-container-label
40mg-500s-container-label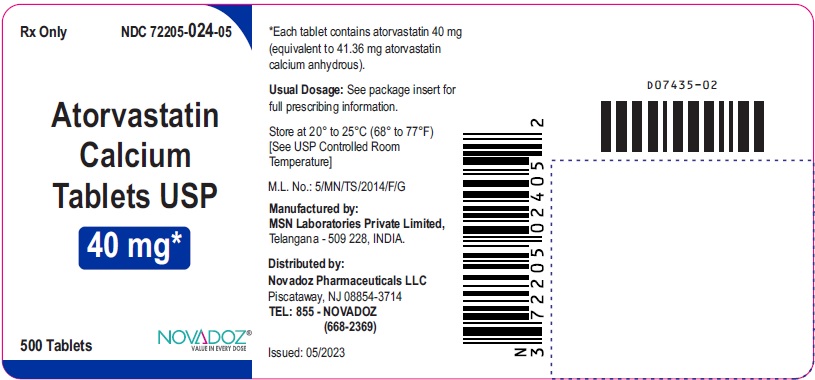
40mg-1000s-container-label

80mg-90s-container-label
80mg-500s-container-label
80mg-1000s-container-label










