NDC Code(s) : 72205-183-01
Packager : Novadoz Pharmaceuticals LLC
Category : Human Prescription Drug Label
DEA Schedule : none
Marketing Status : New Drug Application
INGREDIENTS AND APPEARANCE
| Bortezomib Bortezomib INJECTION, POWDER, LYOPHILIZED, FOR SOLUTION | ||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
| LABELER - Novadoz Pharmaceuticals LLC(081109687) |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
| MSN LABORATORIES PRIVATE LIMITED | 650786952 | ANALYSIS(72205-183), MANUFACTURE(72205-183) | |
PRINCIPAL DISPLAY PANEL
Bortezomib-Carton-ouside-Label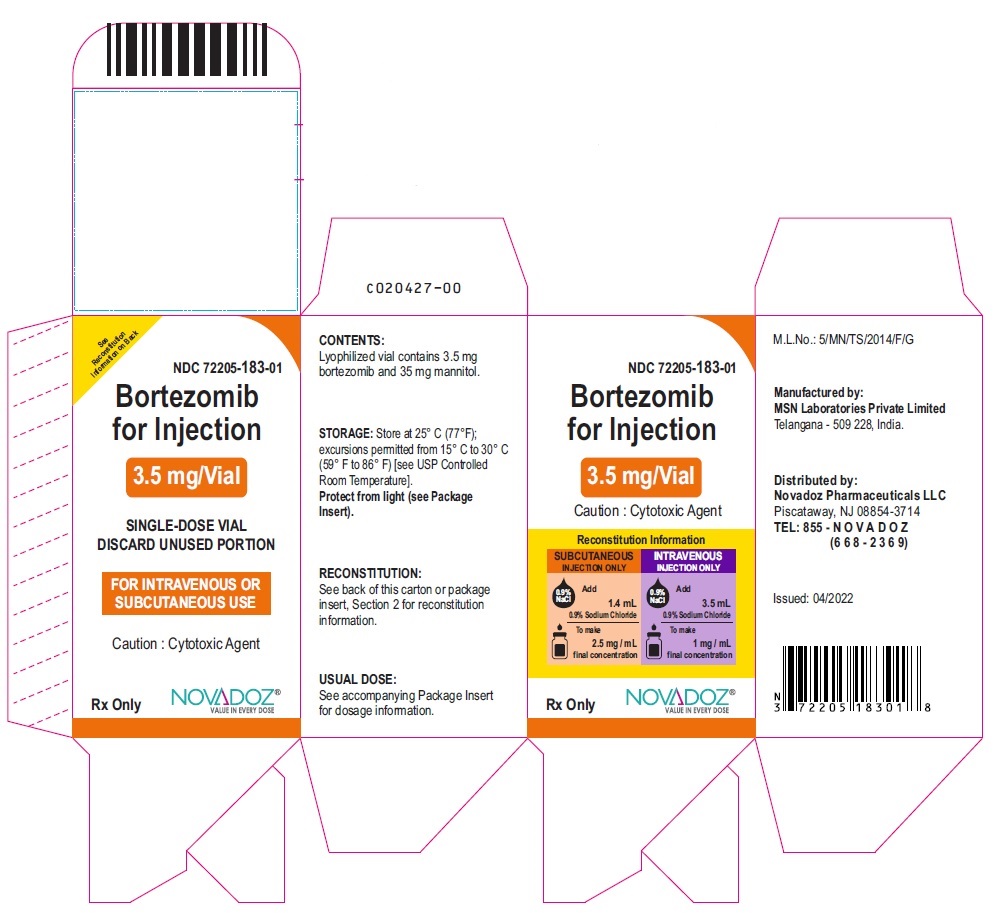
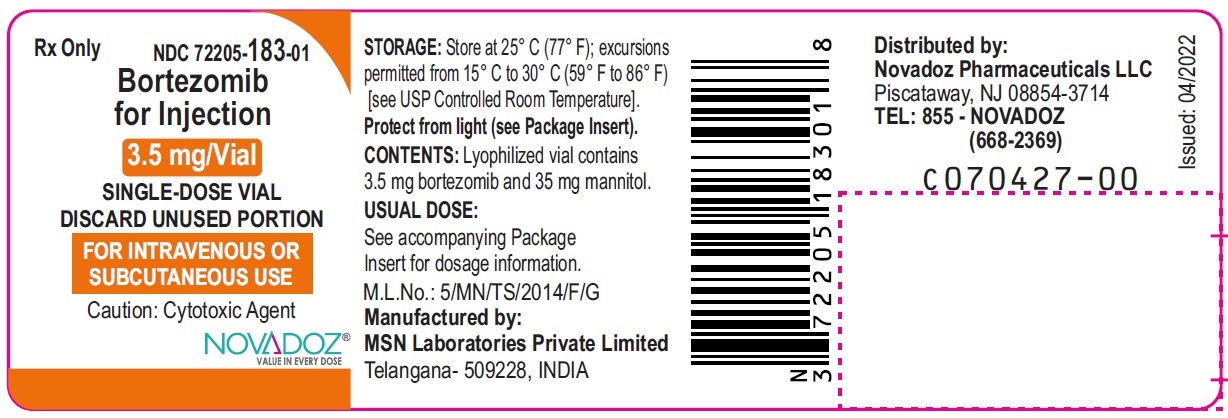
Bortezomib-Vial-Label
Bortezomib-intravenous-sticker-label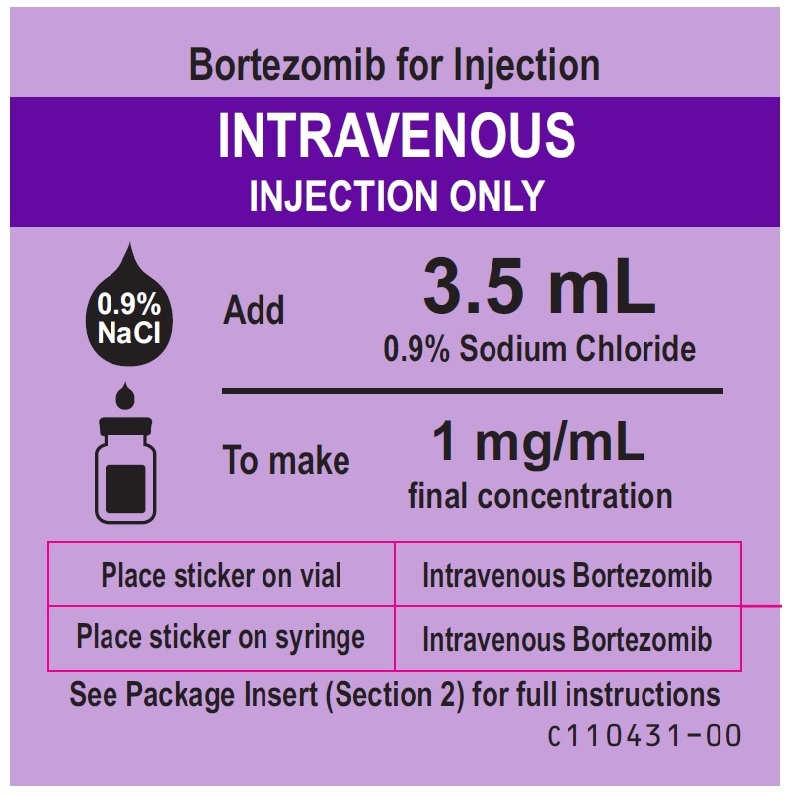
Bortezomib-subcutaneous-sticker-label










