NDC Code(s) : 72485-630-05
Packager : ARMAS PHARMACEUTICALS INC.
Category : HUMAN PRESCRIPTION DRUG LABEL
DEA Schedule : none
Marketing Status : New Drug Application
INGREDIENTS AND APPEARANCE
| Loteprednol EtabonateLoteprednol Etabonate GEL | ||||||||||||||||||||||||
|
||||||||||||||||||||||||
|
||||||||||||||||||||||||
|
||||||||||||||||||||||||
|
||||||||||||||||||||||||
|
||||||||||||||||||||||||
| LABELER - ARMAS PHARMACEUTICALS INC.(098405973) |
| REGISTRANT - SENTISS AG(486920486) |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
| Ophtapharm AG | 482198285 | manufacture(72485-630), label(72485-630), pack(72485-630), analysis(72485-630) | |
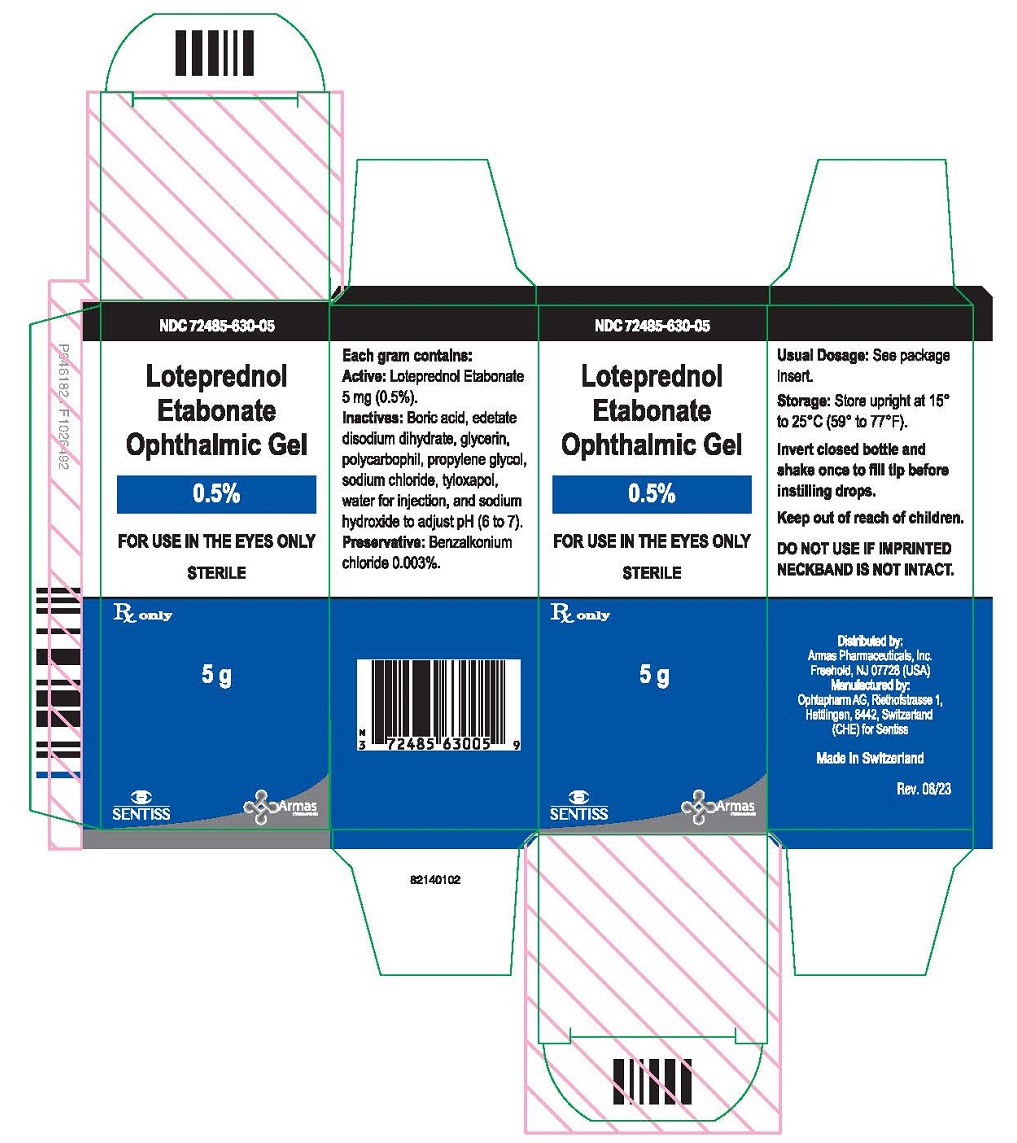
PRINCIPAL DISPLAY PANEL
Principal Display Panel Text for Container Label:
NDC 72485-630-05
Loteprednol
Etabonate
Ophthalmic Gel
0.5%
FOR USE IN THE EYES ONLY
Rx only Sterile 5 g

PRINCIPAL DISPLAY PANEL
Principal Display Panel Text for Carton Label:
NDC 72485-630-05
Loteprednol
Etabonate
Ophthalmic Gel
0.5%
FOR USE IN THE EYES ONLY
Sterile
5 g
Rx only