NDC Code(s) : 72485-634-05, 72485-634-10
Packager : ARMAS PHARMACEUTICALS INC
Category : HUMAN PRESCRIPTION DRUG LABEL
DEA Schedule : none
Marketing Status : New Drug Application
INGREDIENTS AND APPEARANCE
| Brimonidine Tartrate/Timolol MaleateBrimonidine Tartrate and Timolol Maleate SOLUTION/ DROPS | ||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
| LABELER - ARMAS PHARMACEUTICALS INC(098405973) |
| REGISTRANT - SENTISS AG(486920486) |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
| Ophtapharm AG | 482198285 | label(72485-634), manufacture(72485-634), pack(72485-634) | |
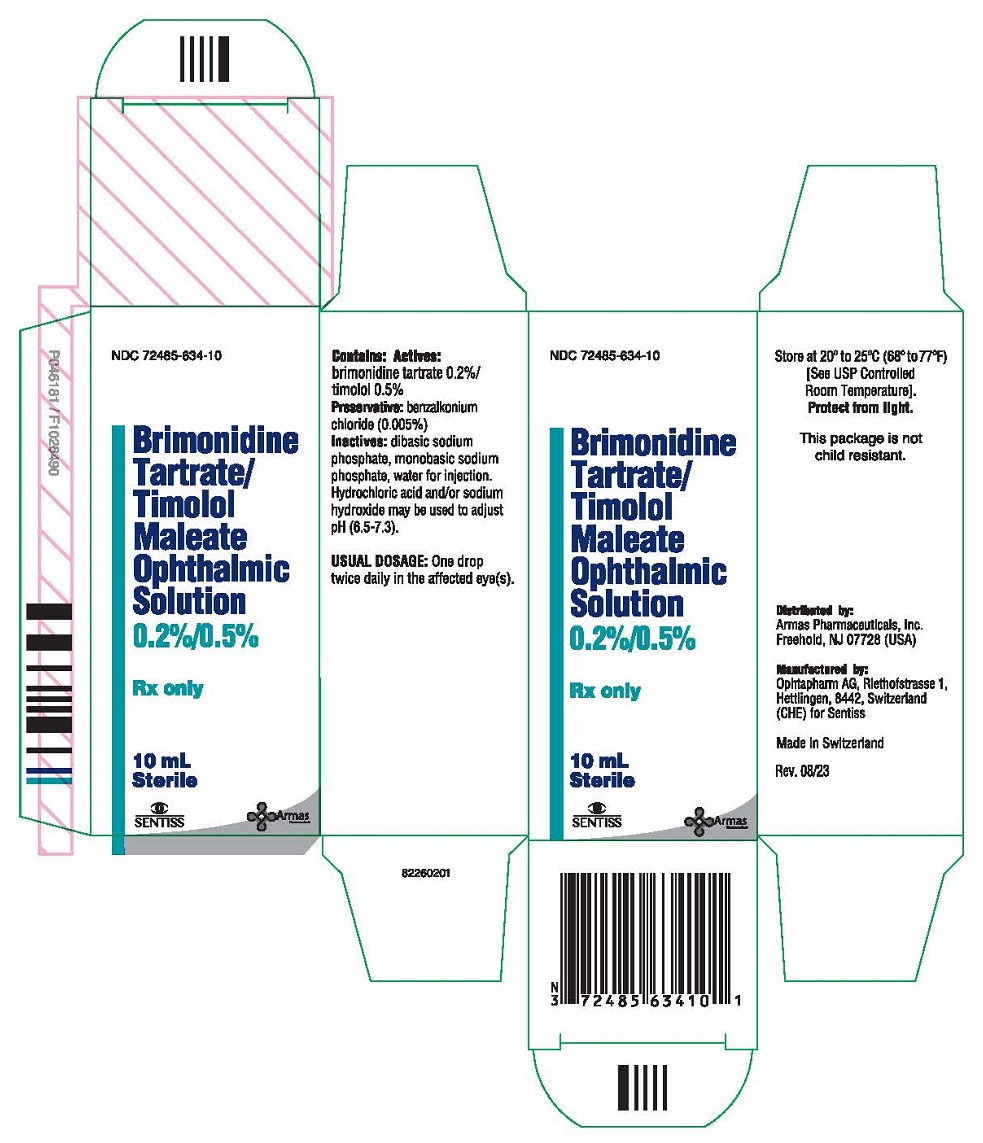
PRINCIPAL DISPLAY PANEL
NDC 72485-634-05
Brimonidine Tartrate/Timolol Maleate Ophthalmic Solution
0.2%/0.5%
Sterile
Rx only
5 mL


PRINCIPAL DISPLAY PANEL
NDC 72485-634-10
Brimonidine Tartrate/Timolol Maleate Ophthalmic Solution
0.2%/0.5%
Sterile
Rx only
10 mL