NDC Code(s) : 72606-001-07, 72606-001-03, 72606-001-08
Packager : CELLTRION USA, INC.
Category : HUMAN PRESCRIPTION DRUG LABEL
DEA Schedule : none
Marketing Status : New Drug Application
INGREDIENTS AND APPEARANCE
| linezolidlinezolid TABLET | ||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
| LABELER - CELLTRION USA, INC.(116587378) |
| REGISTRANT - CELLTRION, INC.(688836030) |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
| Celltrion Pharm, Inc. | 689687234 | manufacture(72606-001), analysis(72606-001), pack(72606-001), label(72606-001) | |
PRINCIPAL DISPLAY PANEL
Rx only NDC 72606-001-03
Linezolid Tablets
600 mg
30 Unit-dose (3×10) Tablets Celltrion USA, INC.
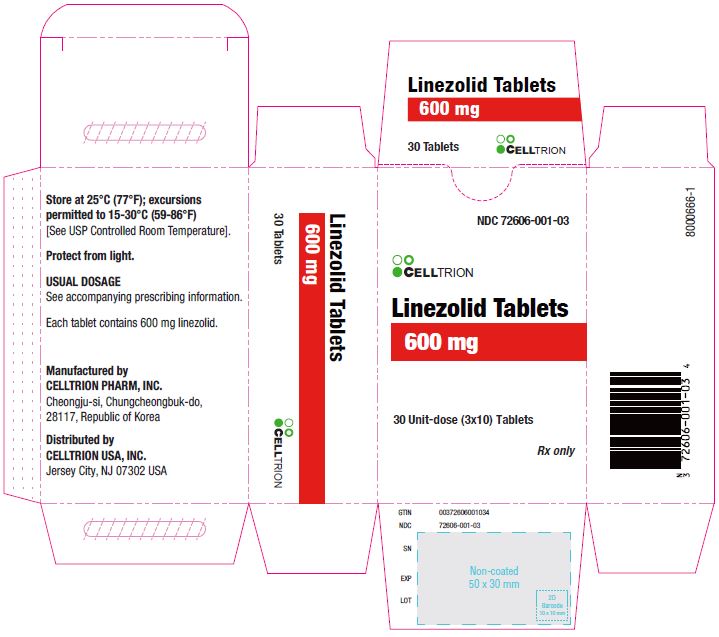
Rx only NDC 72606-001-07
Linezolid Tablets
600 mg
20 Tablets Celltrion USA, INC.

Rx only NDC 72606-001-08
Linezolid Tablets
600 mg
30 Tablets Celltrion USA, INC.










