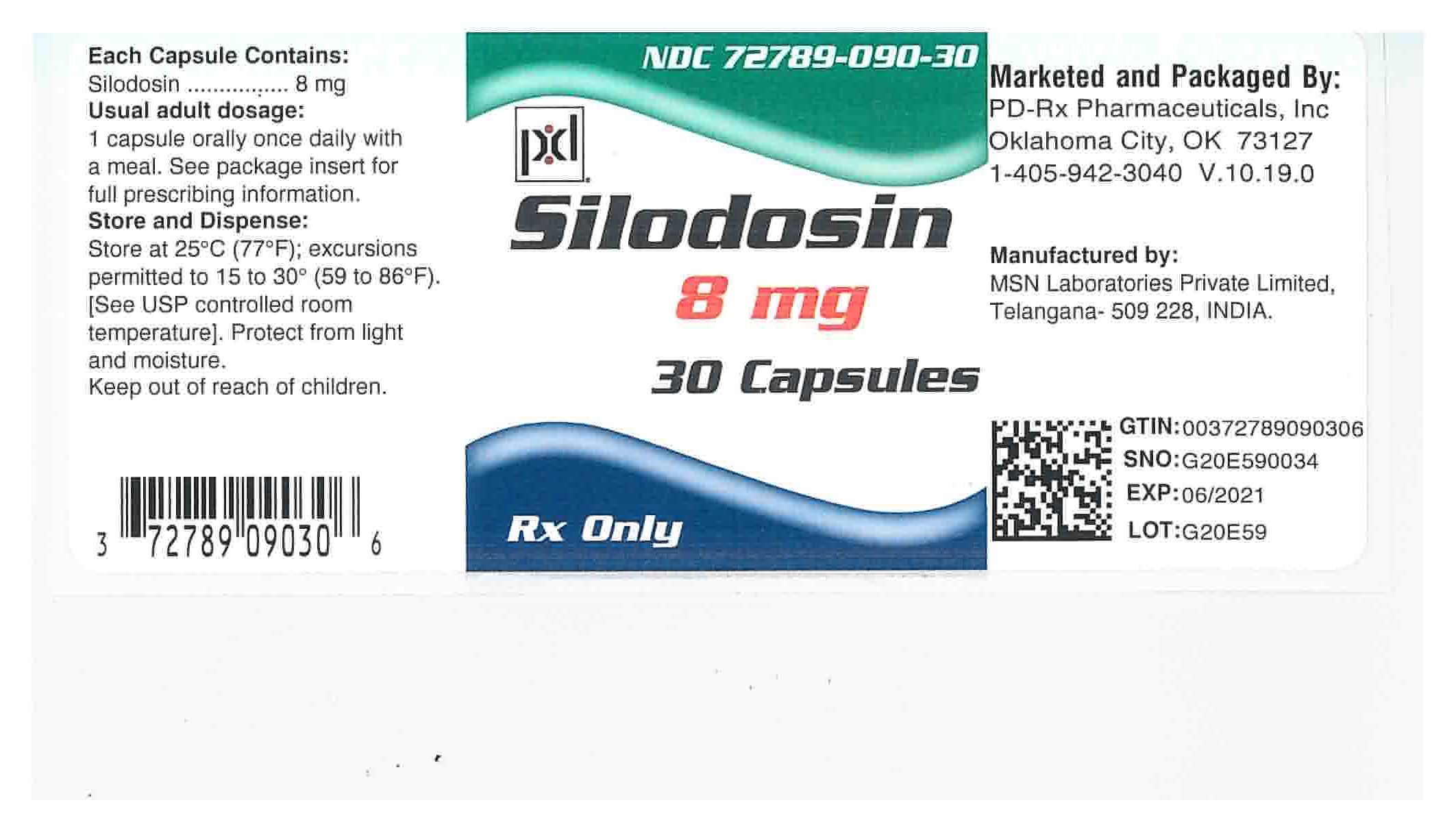
NDC Code(s) : 72789-090-30, 72789-090-90
Packager : PD-Rx Pharmaceuticals, Inc.
Category : HUMAN PRESCRIPTION DRUG LABEL
DEA Schedule : none
Marketing Status : New Drug Application
INGREDIENTS AND APPEARANCE
| SILODOSINSILODOSIN CAPSULE | ||||||||||||||||||||||||||
|
||||||||||||||||||||||||||
|
||||||||||||||||||||||||||
|
||||||||||||||||||||||||||
|
||||||||||||||||||||||||||
|
||||||||||||||||||||||||||
|
||||||||||||||||||||||||||
| LABELER - PD-Rx Pharmaceuticals, Inc.(156893695) |
| REGISTRANT - PD-Rx Pharmaceuticals, Inc.(156893695) |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
| PD-Rx Pharmaceuticals, Inc. | 156893695 | repack(72789-090) | |
PRINCIPAL DISPLAY PANEL