NDC Code(s) : 72819-181-03, 72819-182-09, 72819-183-09, 72819-184-09
Packager : Archis Pharma LLC
Category : HUMAN PRESCRIPTION DRUG LABEL
DEA Schedule : none
Marketing Status : New Drug Application
INGREDIENTS AND APPEARANCE
| Valsartanvalsartan TABLET, COATED | ||||||||||||||||||||||||
|
||||||||||||||||||||||||
|
||||||||||||||||||||||||
|
||||||||||||||||||||||||
|
||||||||||||||||||||||||
|
||||||||||||||||||||||||
|
||||||||||||||||||||||||
| Valsartanvalsartan TABLET, COATED | ||||||||||||||||||||||||
|
||||||||||||||||||||||||
|
||||||||||||||||||||||||
|
||||||||||||||||||||||||
|
||||||||||||||||||||||||
|
||||||||||||||||||||||||
|
||||||||||||||||||||||||
| Valsartanvalsartan TABLET, COATED | ||||||||||||||||||||||||
|
||||||||||||||||||||||||
|
||||||||||||||||||||||||
|
||||||||||||||||||||||||
|
||||||||||||||||||||||||
|
||||||||||||||||||||||||
|
||||||||||||||||||||||||
| Valsartanvalsartan TABLET, COATED | ||||||||||||||||||||||||
|
||||||||||||||||||||||||
|
||||||||||||||||||||||||
|
||||||||||||||||||||||||
|
||||||||||||||||||||||||
|
||||||||||||||||||||||||
|
||||||||||||||||||||||||
| LABELER - Archis Pharma LLC(026836212) |
| REGISTRANT - Square Pharmaceuticals Ltd.(731487153) |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
| Square Pharmaceuticals Ltd. | 850366520 | manufacture(72819-181, 72819-182, 72819-183, 72819-184) | |
PRINCIPAL DISPLAY PANEL
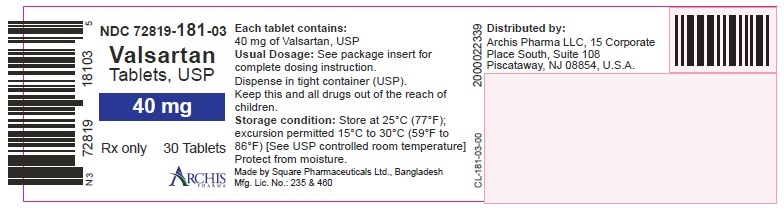
NDC 72819-181-03
Valsartan
Tablets, USP
40 mg
Rx only 30 Tablets
Each tablet contains:
Valsartan, USP 40 mg
Usual Dosage: See packaging insert
for complete dosaging instruction.
Dispense in tight container
Keep this and all drugs out of
the reach of children.
Storage condition: Store at 25 oC (77 oF).
excursion permitted 15 oC TO 30 oC (59 oF TO 86 oF)
[See USP controlled room temperature]
Protect from moisture.
Made by:
Square Pharmaceuticals Ltd.
Bangladesh.
Mfg. Lic. No.: 235 & 460
Distributed by:
Archis Pharma LLC
15 Corporate Place South, Suite 108
Piscataway, NJ 08854, U.S.A.
PRINCIPAL DISPLAY PANEL
NDC 72819-182-09
Valsartan
Tablets, USP
80 mg
Rx only 90 Tablets
Each tablet contains:
Valsartan, USP 80 mg
Usual Dosage: See packaging insert
for complete dosaging instruction.
Dispense in tight container
Keep this and all drugs out of
the reach of children.
Storage condition: Store at 25 oC (77 oF).
excursion permitted 15 oC TO 30 oC (59 oF TO 86 oF)
[See USP controlled room temperature]
Protect from moisture.
Distributed by:
Archis Pharma LLC
15 Corporate Place South, Suite 108
Piscataway, NJ 08854, U.S.A.

PRINCIPAL DISPLAY PANEL
NDC 72819-183-09
Valsartan
Tablets, USP
160 mg
Rx only 90 Tablets
Each tablet contains:
Valsartan, USP 160 mg
Usual Dosage: See packaging insert
for complete dosaging instruction.
Dispense in tight container
Keep this and all drugs out of
the reach of children.
Storage condition: Store at 25 oC (77 oF).
excursion permitted 15 oC TO 30 oC (59 oF TO 86 oF)
[See USP controlled room temperature]
Protect from moisture.
Distributed by:
Archis Pharma LLC
15 Corporate Place South, Suite 108
Piscataway, NJ 08854, U.S.A.
Made by: Square Pharmaceuticals Ltd.
Bangladesh.
Mfg. Lic. No.: 235 & 460

PRINCIPAL DISPLAY PANEL
NDC 72819-184-09
Valsartan
Tablets, USP
320 mg
Rx only 90 Tablets
Each tablet contains:
Valsartan, USP 320 mg
Usual Dosage: See packaging insert
for complete dosaging instruction.
Dispense in tight container
Keep this and all drugs out of
the reach of children.
Storage condition: Store at 25 oC (77 oF).
excursion permitted 15 oC TO 30 oC (59 oF TO 86 oF)
[See USP controlled room temperature]
Protect from moisture
Distributed by:
Archis Pharma LLC
15 Corporate Place South, Suite 108
Piscataway, NJ 08854, U.S.A.
Made by: Square Pharmaceuticals Ltd.
Bangladesh.
Mfg. Lic. No.: 235 & 460










