NDC Code(s) : 72888-096-18, 72888-096-19, 72888-096-34
Packager : Advagen Pharma Ltd
Category : HUMAN PRESCRIPTION DRUG LABEL
DEA Schedule : none
Marketing Status : New Drug Application
INGREDIENTS AND APPEARANCE
| Dihydroergotamine mesylateDihydroergotamine mesylate SPRAY | |||||||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||||||
| LABELER - Advagen Pharma Ltd(051627256) |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
| Mipharm, S.p.A. | 514042399 | manufacture(72888-096), analysis(72888-096), pack(72888-096), label(72888-096) | |
PRINCIPAL DISPLAY PANEL
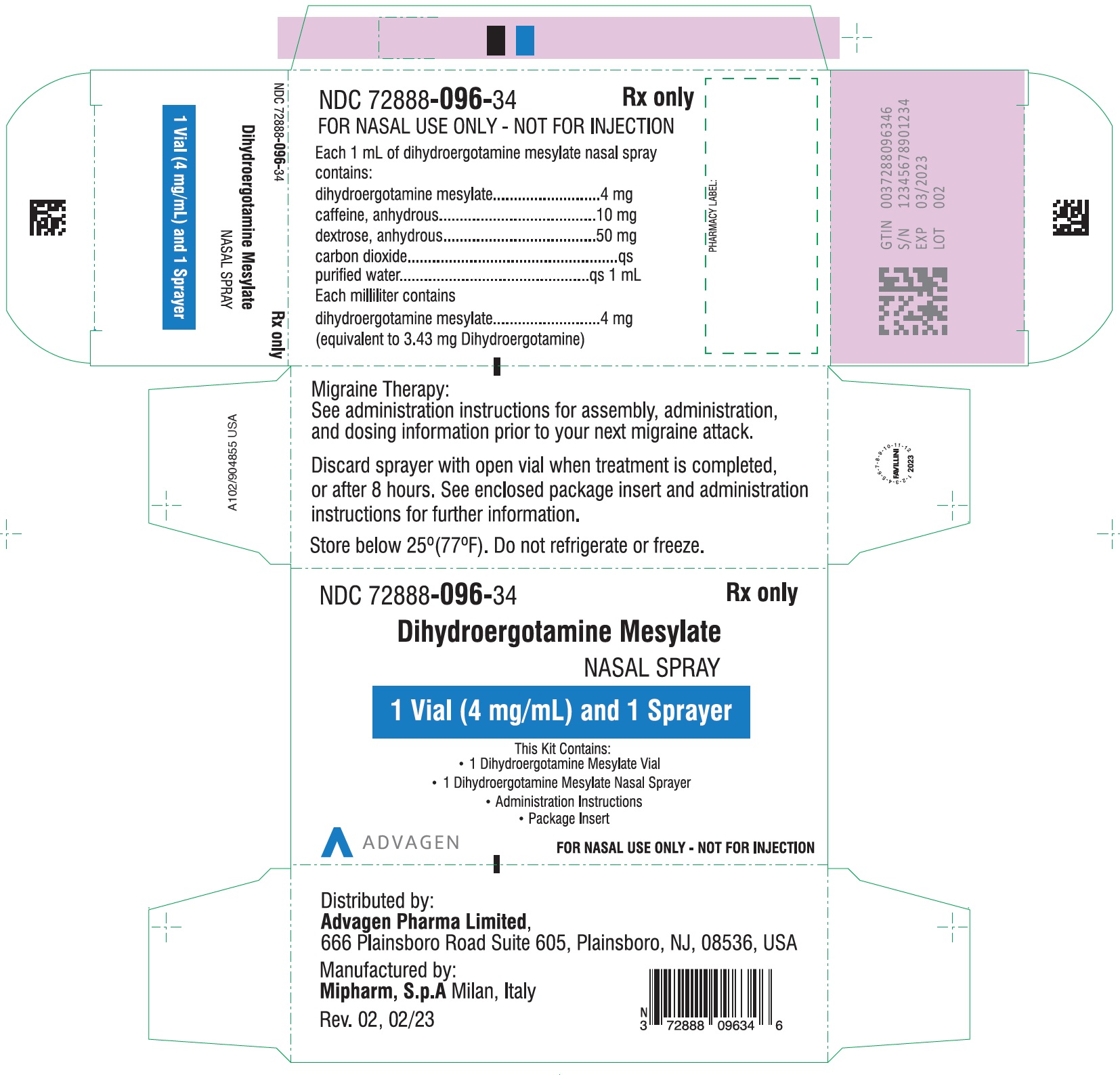
Dihydroergotamine Mesylate Nasal Spray - NDC 72888-096-18 - 1 Unit (1mL Vial and 1 Sprayer) in 1 Carton Label
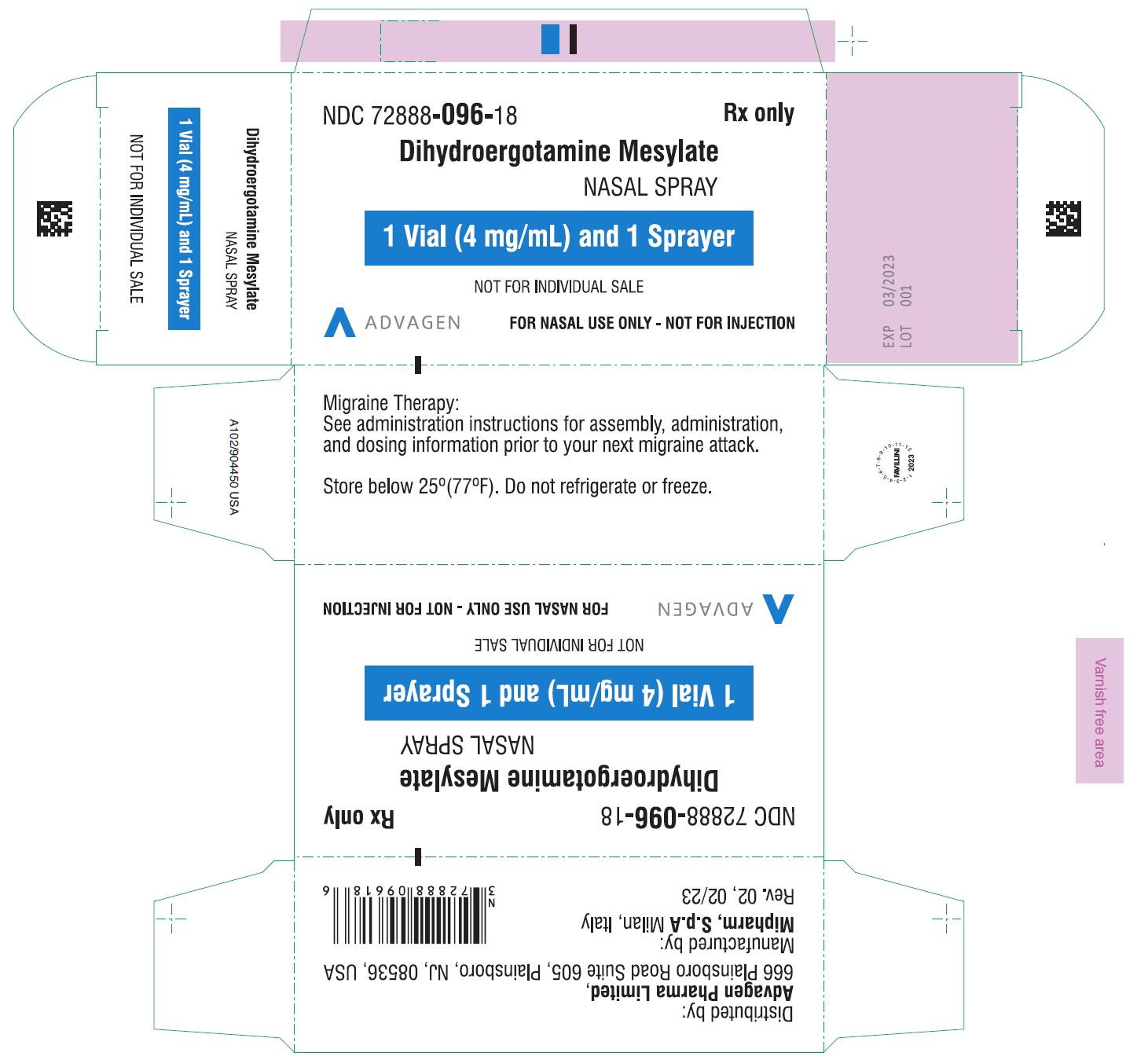
Dihydroergotamine Mesylate Nasal Spray - NDC 72888-096-18 - 1mL Vial Container Label

Dihydroergotamine Mesylate Nasal Spray - NDC 72888-096-19 - 8 Unit's (Each unit contains 1 Vial and 1 Sprayer) in 1 Carton Label
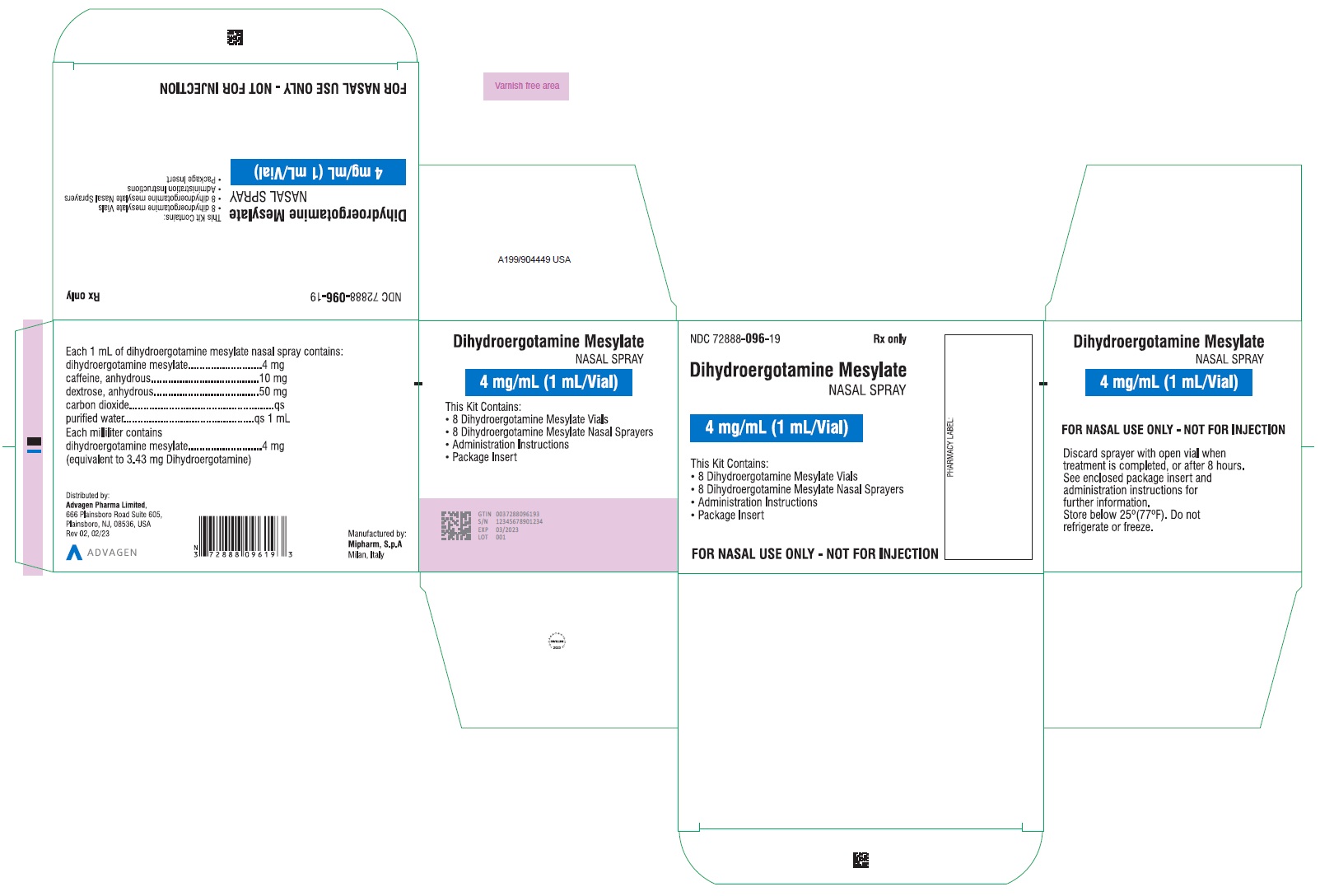
Dihydroergotamine Mesylate Nasal Spray - NDC 72888-096-34 - 1mL Vial and 1 Sprayer in 1 Carton Label