NDC Code(s) : 73015-001-01, 73015-001-02, 73015-001-03, 73015-001-04, 73015-001-05, 73015-001-06, 73015-001-07, 73015-001-08, 73015-001-09, 73015-001-10
Packager : HangZhou YangYi Daily Cosmetics Technology Co., Ltd.
Category : HUMAN OTC DRUG LABEL
DEA Schedule : none
Marketing Status : New Drug Application
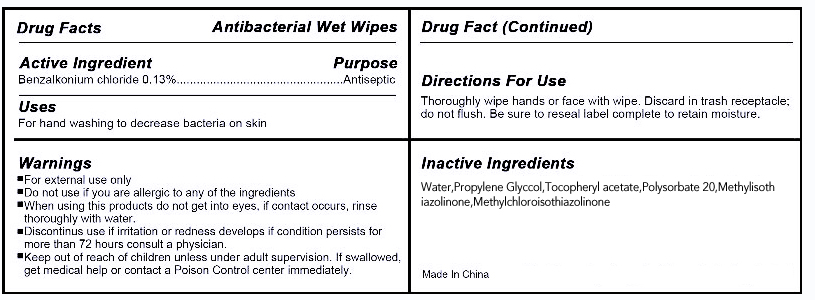
INGREDIENTS AND APPEARANCE
| Antibacterial Wet Wipesbenzalkonium chloride SWAB | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| LABELER - HangZhou YangYi Daily Cosmetics Technology Co., Ltd.(554479892) |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
| HangZhou YangYi Daily Cosmetics Technology Co., Ltd. | 554479892 | manufacture(73015-001) | |
PRINCIPAL DISPLAY PANEL