NDC Code(s) : 76333-264-26, 76333-264-01, 76333-264-17, 76333-265-26, 76333-265-01, 76333-265-17
Packager : Orient Pharma Co.,Ltd Yunlin Plant
Category : HUMAN PRESCRIPTION DRUG LABEL
DEA Schedule : none
Marketing Status : New Drug Application
INGREDIENTS AND APPEARANCE
| VANCOMYCIN HYDROCHLORIDEVANCOMYCIN HYDROCHLORIDE CAPSULE | |||||||||||||||||||||||||
|
|||||||||||||||||||||||||
|
|||||||||||||||||||||||||
|
|||||||||||||||||||||||||
|
|||||||||||||||||||||||||
|
|||||||||||||||||||||||||
|
|||||||||||||||||||||||||
| VANCOMYCIN HYDROCHLORIDEVANCOMYCIN HYDROCHLORIDE CAPSULE | |||||||||||||||||||||||||
|
|||||||||||||||||||||||||
|
|||||||||||||||||||||||||
|
|||||||||||||||||||||||||
|
|||||||||||||||||||||||||
|
|||||||||||||||||||||||||
|
|||||||||||||||||||||||||
| LABELER - Orient Pharma Co.,Ltd Yunlin Plant(658849810) |
| REGISTRANT - Orient Pharma Co.,Ltd Yunlin Plant(658849810) |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
| Orient Pharma Co.,Ltd | 658849810 | manufacture(76333-264, 76333-265) | |
PRINCIPAL DISPLAY PANEL
Vancomycin Hydrochloride Capsules USP, Equiv. to 125 mg vancomycin NDC 76333-264-26 Rx Only 20 Capsules
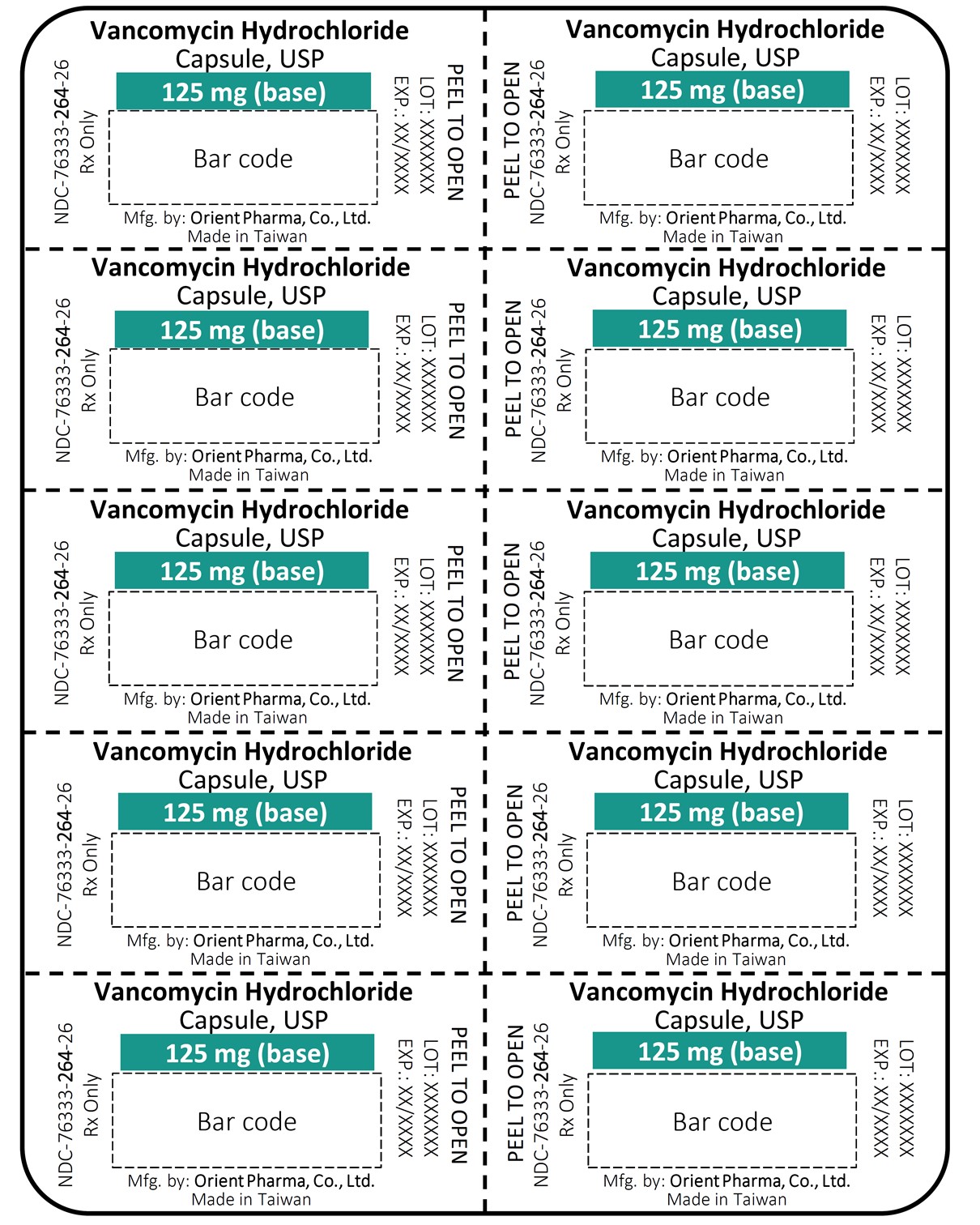

Vancomycin Hydrochloride Capsules USP, Equiv. to 250 mg vancomycin NDC 76333-265-26 Rx Only 20 Capsules


Vancomycin Hydrochloride Capsules USP, Equiv. to 125 mg vancomycin NDC 76333-264-17 Rx Only 50 Capsules
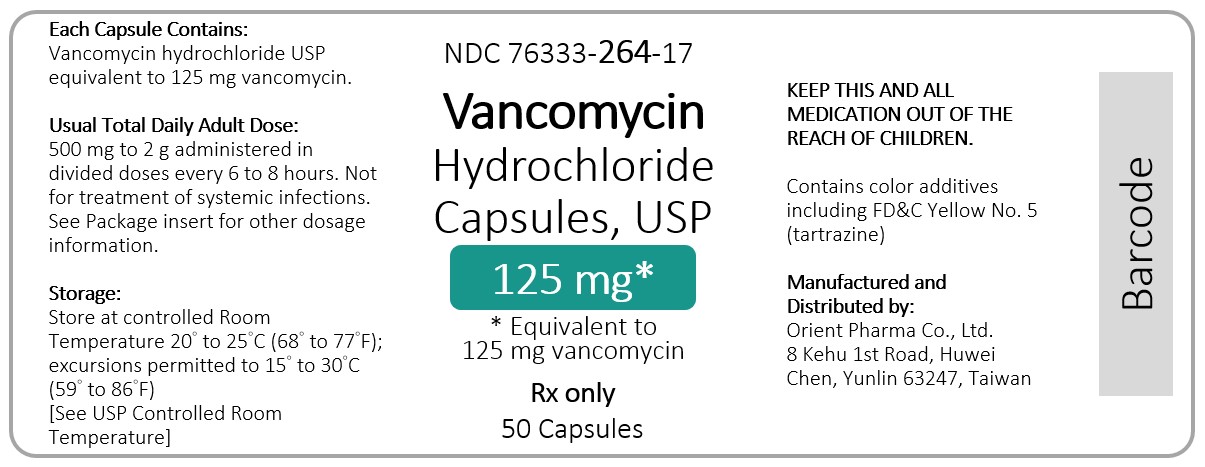
Vancomycin Hydrochloride Capsules USP, Equiv. to 250 mg vancomycin NDC 76333-265-17 Rx Only 50 Capsules










