NDC Code(s) : 76348-412-04
Packager : Renu Laboratories, Inc.
Category : HUMAN PRESCRIPTION DRUG LABEL
DEA Schedule : none
Marketing Status : New Drug Application
INGREDIENTS AND APPEARANCE
| CliXit (TM) acne eraserAcne Pen CREAM | ||||||||||||||||||||||||
|
||||||||||||||||||||||||
|
||||||||||||||||||||||||
|
||||||||||||||||||||||||
|
||||||||||||||||||||||||
|
||||||||||||||||||||||||
PRINCIPAL DISPLAY PANEL
.jpg)
%20(263x640).jpg)
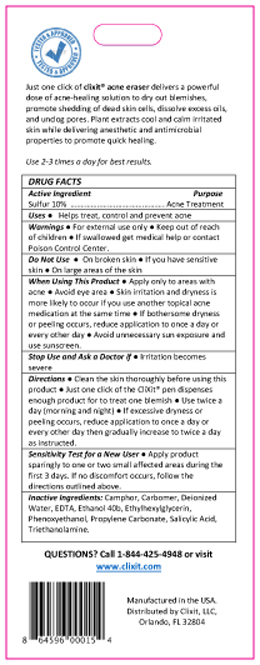
.jpg)
%20(640x90)%20copy%20(2).jpg)









