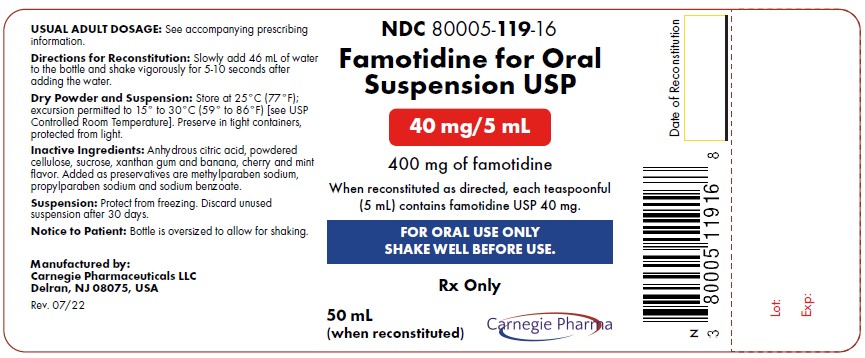
NDC Code(s) : 80005-119-16
Packager : Carnegie Pharmaceuticals LLC
Category : HUMAN PRESCRIPTION DRUG LABEL
DEA Schedule : none
Marketing Status : New Drug Application
INGREDIENTS AND APPEARANCE
| FAMOTIDINEFAMOTIDINE POWDER, FOR SUSPENSION | ||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
| LABELER - Carnegie Pharmaceuticals LLC(079839141) |
| REGISTRANT - Carnegie Pharmaceuticals LLC(079839141) |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
| Carnegie Pharmaceuticals LLC | 079839141 | manufacture(80005-119), analysis(80005-119), pack(80005-119), label(80005-119) | |
PRINCIPAL DISPLAY PANEL
Packet Label: