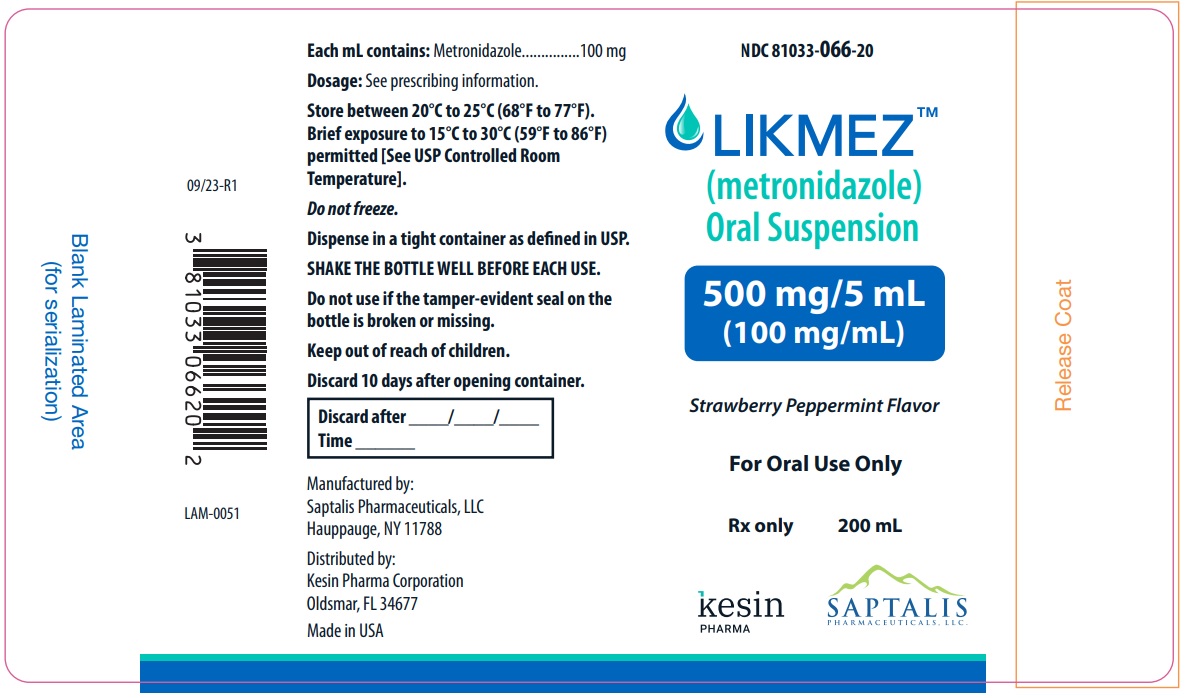
NDC Code(s) : 81033-066-20
Packager : Kesin Pharma Corporation
Category : HUMAN PRESCRIPTION DRUG LABEL
DEA Schedule : none
Marketing Status : New Drug Application
INGREDIENTS AND APPEARANCE
| LIKMEZMetronidazole Oral SUSPENSION | ||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||
| LABELER - Kesin Pharma Corporation(117447816) |
| REGISTRANT - Saptalis Pharmaceuticals, LLC(081154447) |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
| Saptalis Pharmaceuticals, LLC | 081154447 | manufacture(81033-066) | |
PRINCIPAL DISPLAY PANEL
NDC 81033-066-20
LIKMEZTM
(metronidazole) Oral Supension
500 mg/mL
(100 mg/mL)
Strawberry Peppermint Flavor
For Oral Use Only
Rx only
200 mL