NDC Code(s) : 86026-001-21, 86026-001-23, 86026-001-26, 86026-002-21, 86026-002-23, 86026-002-26, 86026-003-21, 86026-003-23, 86026-003-26
Packager : Aratana Therapeutics, Inc.
Category : HUMAN PRESCRIPTION DRUG LABEL
DEA Schedule : none
Marketing Status : New Drug Application
INGREDIENTS AND APPEARANCE
| Galliprantgrapiprant TABLET | |||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Galliprantgrapiprant TABLET | |||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Galliprantgrapiprant TABLET | |||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||
PRINCIPAL DISPLAY PANEL
NDC 86026-001-21
Galliprant®
(grapiprant tablets)
20 mg
For oral use in dogs only
EP4 Prostaglandin Receptor
Antagonist NSAID
Caution: Federal (USA) law
restricts this drug to use by
or on the order of a
licensed veterinarian.
7 Flavored
Tablets
NADA 141-455
Approved by FDA
ARATANA
THERAPEUTICS®

PRINCIPAL DISPLAY PANEL
NDC 86026-001-21
Galliprant®
(grapiprant tablets)
20 mg
For oral use in dogs only
EP4 Prostaglandin Receptor
Antagonist NSAID
Caution: Federal (USA) law
restricts this drug to use by or on
the order of a licensed veterinarian.
7 Flavored Tablets
NADA 141-455
Approved by FDA
ARATANA
THERAPEUTICS®

PRINCIPAL DISPLAY PANEL
NDC 86026-002-21
Galliprant®
(grapiprant tablets)
60 mg
For oral use in dogs only
EP4 Prostaglandin Receptor
Antagonist NSAID
Caution: Federal (USA) law
restricts this drug to use by
or on the order of a
licensed veterinarian.
7 Flavored
Tablets
NADA 141-455
Approved by FDA
ARATANA
THERAPEUTICS®

PRINCIPAL DISPLAY PANEL
NDC 86026-002-21
Galliprant®
(grapiprant tablets)
60 mg
For oral use in dogs only
EP4 Prostaglandin Receptor
Antagonist NSAID
Caution: Federal (USA) law
restricts this drug to use by or on
the order of a licensed veterinarian.
7 Flavored Tablets
NADA 141-455
Approved by FDA
ARATANA
THERAPEUTICS®

PRINCIPAL DISPLAY PANEL
NDC 86026-003-21
Galliprant®
(grapiprant tablets)
100 mg
For oral use in dogs only
EP4 Prostaglandin Receptor
Antagonist NSAID
Caution: Federal (USA) law restricts
this drug to use by or on the order
of a licensed veterinarian.
7 Flavored
Tablets
NADA 141-455
Approved by FDA
ARATANA
THERAPEUTICS®

PRINCIPAL DISPLAY PANEL
NDC 86026-003-21
Galliprant®
(grapiprant tablets)
100 mg
For oral use in dogs only
EP4 Prostaglandin Receptor Antagonist NSAID
Caution: Federal (USA) law restricts this drug to
use by or on the order of a licensed veterinarian.
7 Flavored Tablets
NADA 141-455
Approved by FDA
ARATANA
THERAPEUTICS®

PRINCIPAL DISPLAY PANEL
NDC 86026-001-23
Galliprant®
(grapiprant tablets)
20 mg
For oral use in dogs only
EP4 Prostaglandin Receptor
Antagonist NSAID
Caution: Federal (USA) law
restricts this drug to use by
or on the order of a
licensed veterinarian.
30 Flavored
Tablets
NADA 141-455
Approved by FDA
ARATANA
THERAPEUTICS®

PRINCIPAL DISPLAY PANEL
NDC 86026-001-23
Galliprant®
(grapiprant tablets)
20 mg
For oral use in dogs only
EP4 Prostaglandin Receptor
Antagonist NSAID
Caution: Federal (USA) law
restricts this drug to use by or on
the order of a licensed veterinarian.
30 Flavored Tablets
NADA 141-455
Approved by FDA
ARATANA
THERAPEUTICS®

PRINCIPAL DISPLAY PANEL
NDC 86026-002-23
Galliprant®
(grapiprant tablets)
60 mg
For oral use in dogs only
EP4 Prostaglandin Receptor
Antagonist NSAID
Caution: Federal (USA) law restricts
this drug to use by or on the order
of a licensed veterinarian.
30 Flavored
Tablets
NADA 141-455
Approved by FDA
ARATANA
THERAPEUTICS®
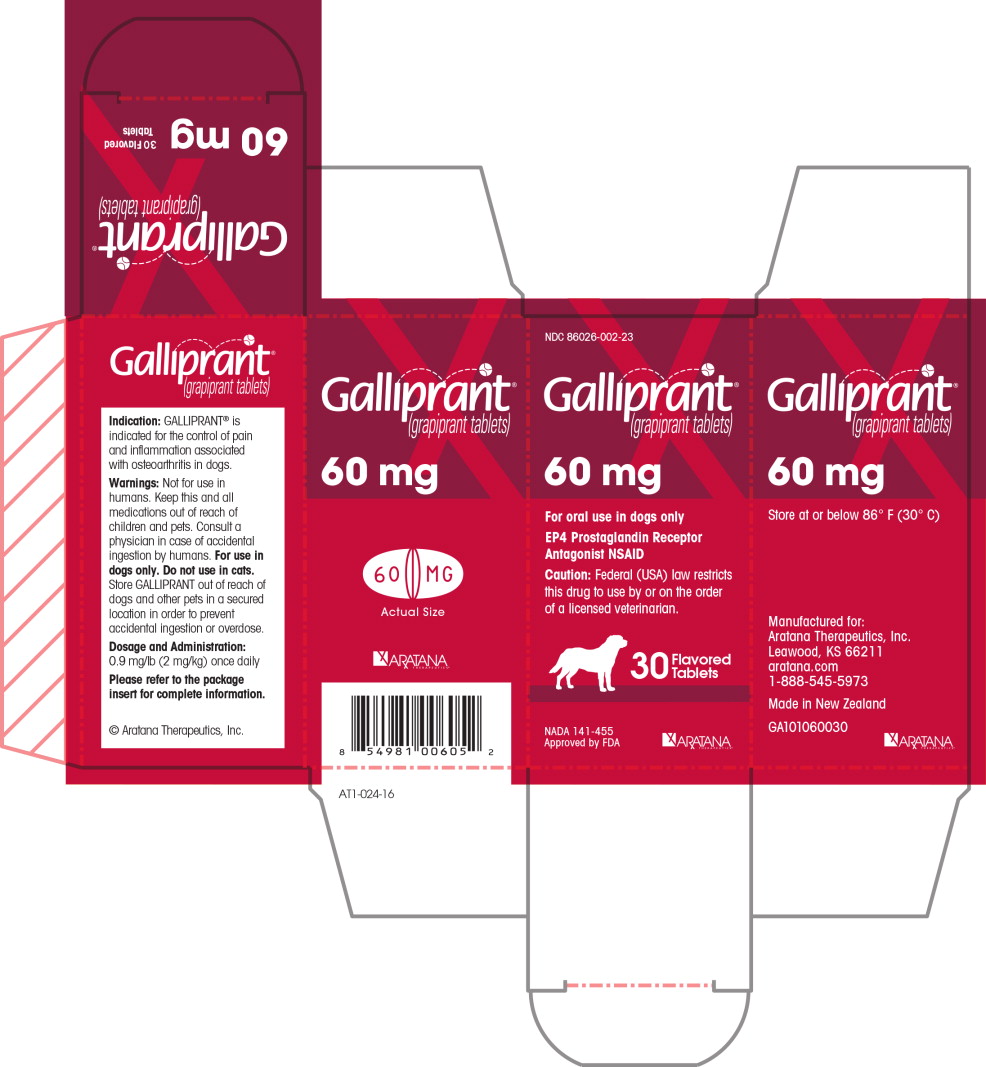
PRINCIPAL DISPLAY PANEL
NDC 86026-002-23
Galliprant®
(grapiprant tablets)
60 mg
For oral use in dogs only
EP4 Prostaglandin Receptor Antagonist NSAID
Caution: Federal (USA) law restricts this drug to
use by or on the order of a licensed veterinarian.
30 Flavored Tablets
NADA 141-455
Approved by FDA
ARATANA
THERAPEUTICS®

PRINCIPAL DISPLAY PANEL
NDC 86026-003-23
Galliprant®
(grapiprant tablets)
100 mg
For oral use in dogs only
EP4 Prostaglandin Receptor
Antagonist NSAID
Caution: Federal (USA) law
restricts this drug to use by or on
the order of a licensed veterinarian.
30 Flavored
Tablets
NADA 141-455
Approved by FDA
ARATANA
THERAPEUTICS®

PRINCIPAL DISPLAY PANEL
NDC 86026-003-23
Galliprant®
(grapiprant tablets)
100 mg
For oral use in dogs only
EP4 Prostaglandin Receptor Antagonist NSAID
Caution: Federal (USA) law restricts this drug to
use by or on the order of a licensed veterinarian.
30 Flavored Tablets
NADA 141-455
Approved by FDA
ARATANA
THERAPEUTICS®
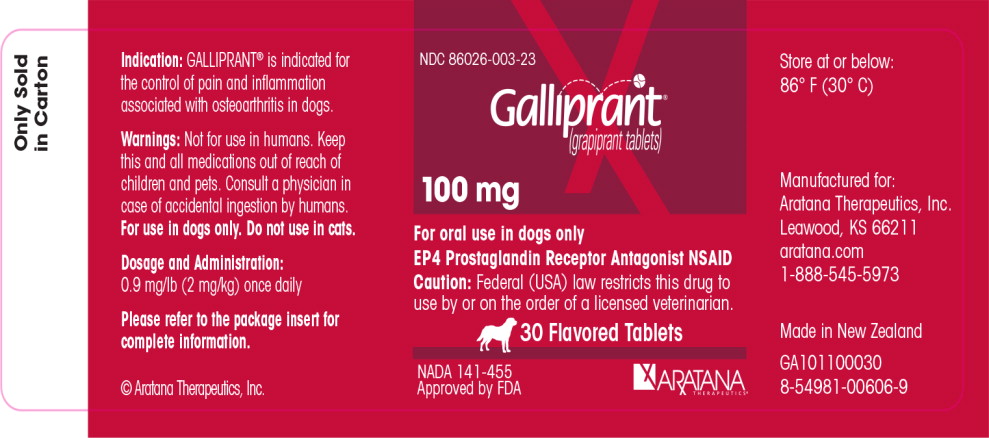
PRINCIPAL DISPLAY PANEL
NDC 86026-001-26
Galliprant®
(grapiprant tablets)
20 mg
For oral use in dogs only
EP4 Prostaglandin Receptor
Antagonist NSAID
Caution: Federal (USA) law restricts
this drug to use by or on the order
of a licensed veterinarian.
90 Flavored
Tablets
NADA 141-455
Approved by FDA
ARATANA
THERAPEUTICS®
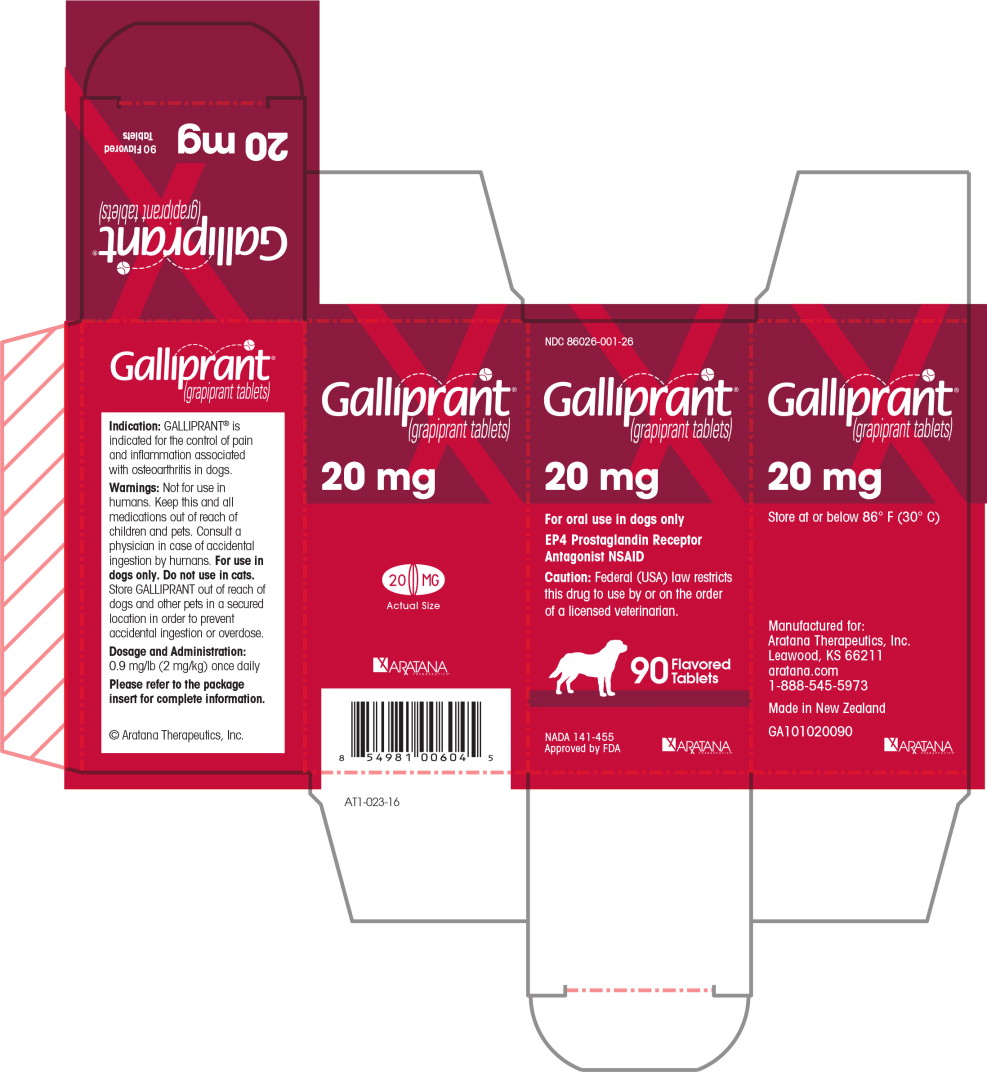
PRINCIPAL DISPLAY PANEL
NDC 86026-001-26
Galliprant®
(grapiprant tablets)
20 mg
For oral use in dogs only
EP4 Prostaglandin Receptor Antagonist NSAID
Caution: Federal (USA) law restricts this drug to
use by or on the order of a licensed veterinarian.
90 Flavored Tablets
NADA 141-455
Approved by FDA
ARATANA
THERAPEUTICS®

PRINCIPAL DISPLAY PANEL
NDC 86026-002-26
Galliprant®
(grapiprant tablets)
60 mg
For oral use in dogs only
EP4 Prostaglandin Receptor
Antagonist NSAID
Caution: Federal (USA) law restricts
this drug to use by or on the order
of a licensed veterinarian.
90 Flavored
Tablets
NADA 141-455
Approved by FDA
ARATANA
THERAPEUTICS®
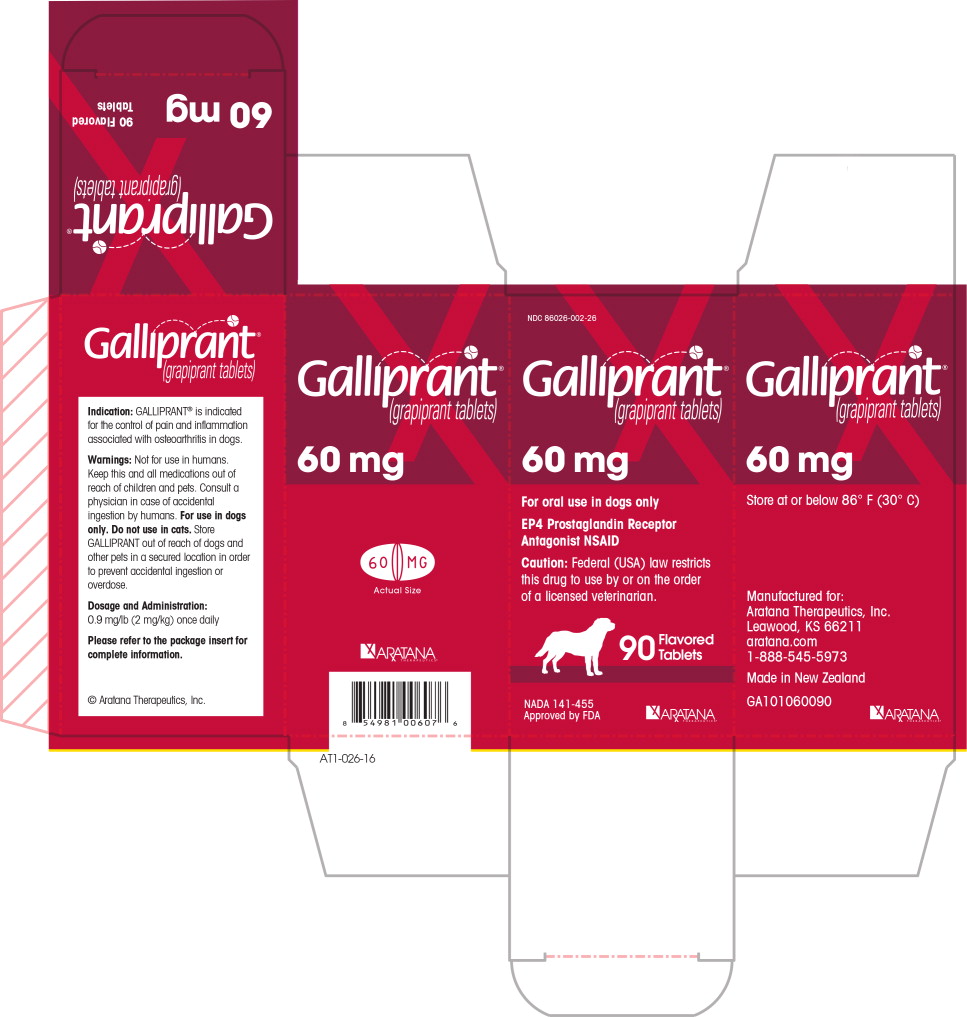
PRINCIPAL DISPLAY PANEL
NDC 86026-002-26
Galliprant®
(grapiprant tablets)
60 mg
For oral use in dogs only
EP4 Prostaglandin Receptor Antagonist NSAID
Caution: Federal (USA) law restricts this drug to use by or
on the order of a licensed veterinarian.
90 Flavored Tablets
NADA 141-455
Approved by FDA
ARATANA
THERAPEUTICS®

PRINCIPAL DISPLAY PANEL
NDC 86026-003-26
Galliprant®
(grapiprant tablets)
100 mg
For oral use in dogs only
EP4 Prostaglandin Receptor
Antagonist NSAID
Caution: Federal (USA) law restricts
this drug to use by or on the order
of a licensed veterinarian.
90 Flavored
Tablets
NADA 141-455
Approved by FDA
ARATANA
THERAPEUTICS®

PRINCIPAL DISPLAY PANEL
NDC 86026-003-26
Galliprant®
(grapiprant tablets)
100 mg
For oral use in dogs only
EP4 Prostaglandin Receptor Antagonist NSAID
Caution: Federal (USA) law restricts this drug to use by or
on the order of a licensed veterinarian.
90 Flavored Tablets
NADA 141-455
Approved by FDA
ARATANA
THERAPEUTICS®










