NDC Code(s) : 98132-252-50
Packager : Orveon Global US LLC
Category : HUMAN OTC DRUG LABEL
DEA Schedule : none
Marketing Status : New Drug Application
INGREDIENTS AND APPEARANCE
| bareMinerals Skinlongevity Vital Power Moisturizer Broad Spectrum SPF 30TITANIUM DIOXIDE and ZINC OXIDE CREAM | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| LABELER - Orveon Global US LLC(118344494) |
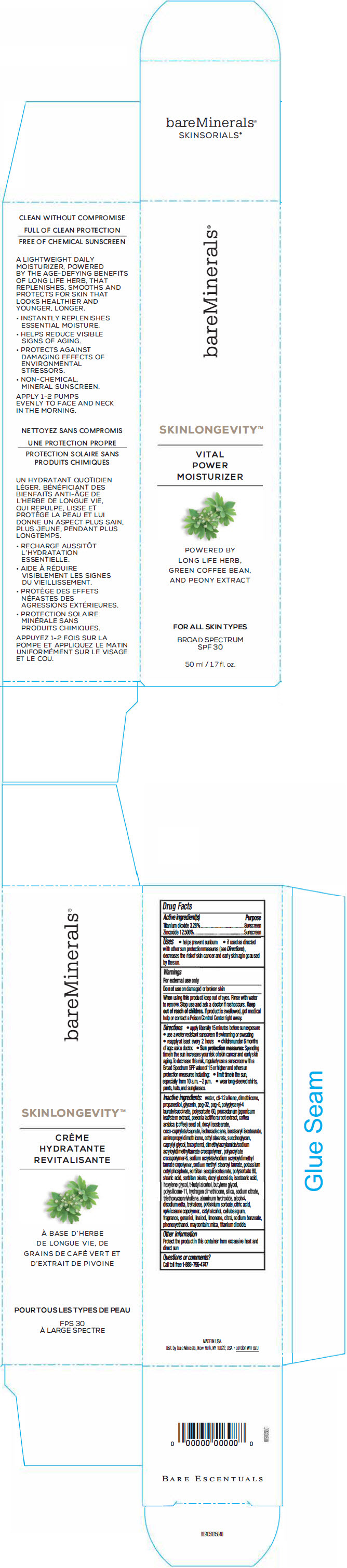
PRINCIPAL DISPLAY PANEL
bareMinerals ®
SKINLONGEVITY™
VITAL
POWER
MOISTURIZER
POWERED BY
LONG LIFE HERB,
GREEN COFFEE BEAN,
AND PEONY EXTRACT
FOR ALL SKIN TYPES
BROAD SPECTRUM
SPF30
50 ml / 1.7 fl. oz.