NDC Code(s) : 98132-751-01, 98132-752-01, 98132-753-01, 98132-754-01, 98132-755-01, 98132-756-01, 98132-757-01, 98132-758-01, 98132-759-01, 98132-760-01
Packager : Orveon Global US, LLC
Category : HUMAN OTC DRUG LABEL
DEA Schedule : none
Marketing Status : New Drug Application
INGREDIENTS AND APPEARANCE
| bareMinerals Complexion Rescue Broad Spectrum SPF 30 Titanium Dioxide LIQUID | ||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||
| bareMinerals Complexion Rescue Broad Spectrum SPF 30 Titanium Dioxide LIQUID | ||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||
| bareMinerals Complexion Rescue Broad Spectrum SPF 30 Titanium Dioxide LIQUID | ||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||
| bareMinerals Complexion Rescue Broad Spectrum SPF 30 Titanium Dioxide LIQUID | ||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||
| bareMinerals Complexion Rescue Broad Spectrum SPF 30 Titanium Dioxide LIQUID | ||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||
| bareMinerals Complexion Rescue Broad Spectrum SPF 30 Titanium Dioxide LIQUID | ||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||
| bareMinerals Complexion Rescue Broad Spectrum SPF 30 Titanium Dioxide LIQUID | ||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||
| bareMinerals Complexion Rescue Broad Spectrum SPF 30 Titanium Dioxide LIQUID | ||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||
| bareMinerals Complexion Rescue Broad Spectrum SPF 30 Titanium Dioxide LIQUID | ||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||
| bareMinerals Complexion Rescue Broad Spectrum SPF 30 Titanium Dioxide LIQUID | ||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||
| LABELER - Orveon Global US, LLC(118344494) |
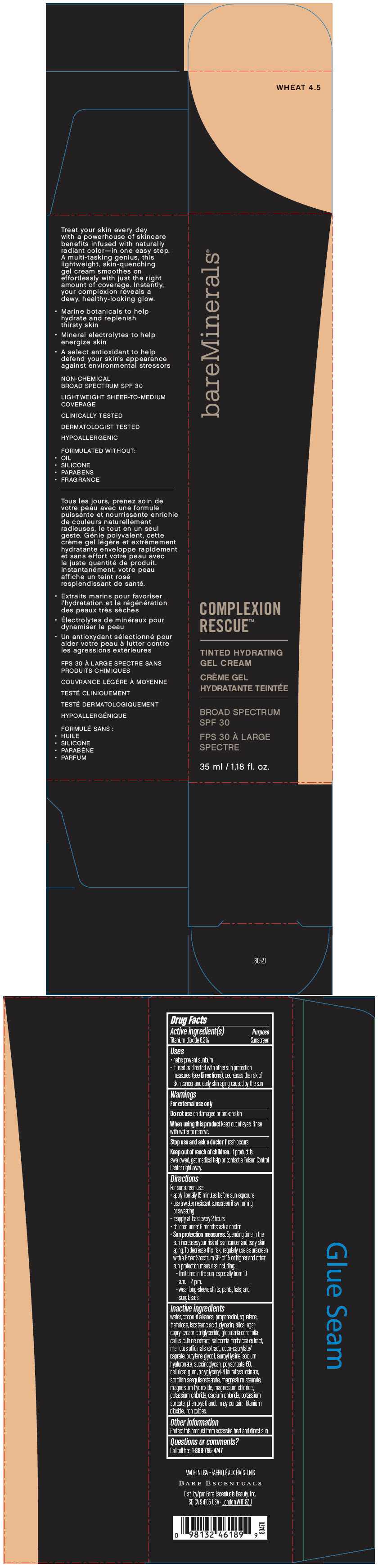
PRINCIPAL DISPLAY PANEL
bareMinerals ®
COMPLEXION
RESCUE™
TINTED HYDRATING
GEL CREAM
BROAD SPECTRUM
SPF 30
35 ml / 1.18 fl. oz.


PRINCIPAL DISPLAY PANEL
bareMinerals ®
COMPLEXION
RESCUE™
TINTED HYDRATING
GEL CREAM
BROAD SPECTRUM
SPF 30
35 ml / 1.18 fl. oz.

PRINCIPAL DISPLAY PANEL
bareMinerals ®
COMPLEXION
RESCUE™
TINTED HYDRATING
GEL CREAM
BROAD SPECTRUM
SPF 30
35 ml / 1.18 fl. oz.

PRINCIPAL DISPLAY PANEL
bareMinerals ®
COMPLEXION
RESCUE™
TINTED HYDRATING
GEL CREAM
BROAD SPECTRUM
SPF 30
35 ml / 1.18 fl. oz.

PRINCIPAL DISPLAY PANEL
bareMinerals ®
COMPLEXION
RESCUE™
TINTED HYDRATING
GEL CREAM
BROAD SPECTRUM
SPF 30
35 ml / 1.18 fl. oz.

PRINCIPAL DISPLAY PANEL
bareMinerals ®
COMPLEXION
RESCUE™
TINTED HYDRATING
GEL CREAM
BROAD SPECTRUM
SPF 30
35 ml / 1.18 fl. oz.

PRINCIPAL DISPLAY PANEL
bareMinerals ®
COMPLEXION
RESCUE™
TINTED HYDRATING
GEL CREAM
BROAD SPECTRUM
SPF 30
35 ml / 1.18 fl. oz.

PRINCIPAL DISPLAY PANEL
bareMinerals ®
COMPLEXION
RESCUE™
TINTED HYDRATING
GEL CREAM
BROAD SPECTRUM
SPF 30
35 ml / 1.18 fl. oz.

PRINCIPAL DISPLAY PANEL
bareMinerals ®
COMPLEXION
RESCUE™
TINTED HYDRATING
GEL CREAM
BROAD SPECTRUM
SPF 30
35 ml / 1.18 fl. oz.

PRINCIPAL DISPLAY PANEL
bareMinerals ®
COMPLEXION
RESCUE™
TINTED HYDRATING
GEL CREAM
BROAD SPECTRUM
SPF 30
35 ml / 1.18 fl. oz.










