04 Feb 2025
// PRESS RELEASE
19 Nov 2024
// PRESS RELEASE
10 Nov 2024
// PRESS RELEASE
 KEY PRODUCTS
KEY PRODUCTS
HealthTech BioActives, leading globally in Flavonoids and Vitamin B12 Derivatives.
About
Industry Trade Show
Not Confirmed
24-26 February, 2026
Global ChemShowGlobal ChemShow
Industry Trade Show
Not Confirmed
19-20 December, 2025
BIO Partnering at JPMBIO Partnering at JPM
Industry Trade Show
Not Confirmed
12-15 January, 2026
CONTACT DETAILS





Events
Webinars & Exhibitions
Industry Trade Show
Not Confirmed
24-26 February, 2026
Global ChemShowGlobal ChemShow
Industry Trade Show
Not Confirmed
19-20 December, 2025
BIO Partnering at JPMBIO Partnering at JPM
Industry Trade Show
Not Confirmed
12-15 January, 2026
CORPORATE CONTENT #SupplierSpotlight
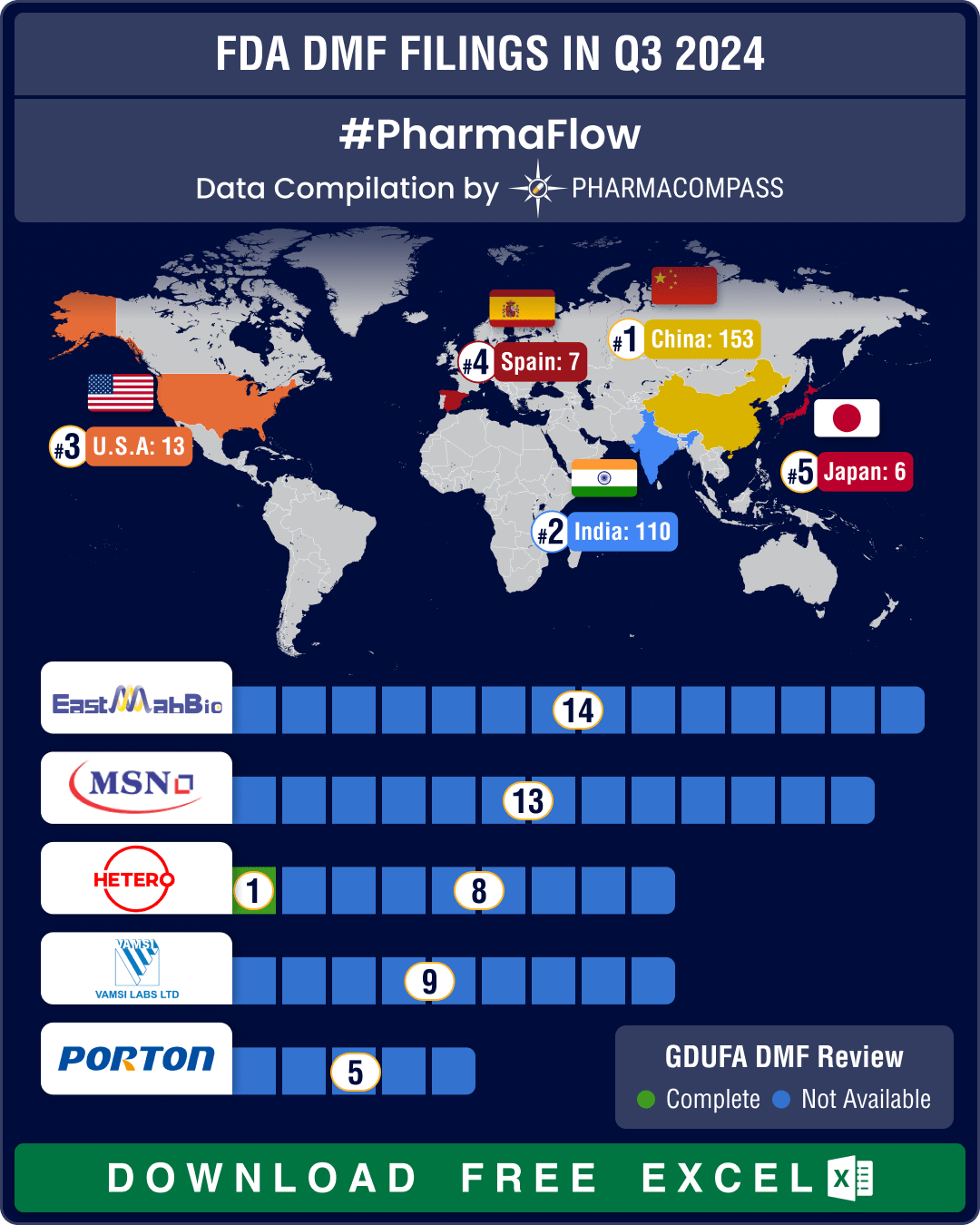
https://www.pharmacompass.com/radio-compass-blog/dmf-filings-hit-all-time-high-in-q3-2024-china-tops-list-with-58-increase-in-type-ii-submissions

04 Feb 2025
// PRESS RELEASE
https://htba.com/htba-strengthens-its-commitment-to-sustainability-and-innovation-with-a-25-millioneuros-investment-in-its-beniel-industrial-center/

19 Nov 2024
// PRESS RELEASE
https://htba.com/htba-to-champion-sensorial-excellence-at-fi-europe-2024-with-spotlight-on-flavonoid-powered-taste-modulation-solutions/

10 Nov 2024
// PRESS RELEASE
https://htba.com/htba-to-champion-sensorial-excellence-at-fi-europe-2024-with-spotlight-on-flavonoid-powered-taste-modulation-solutions/

05 Nov 2024
// PRESS RELEASE
https://htba.com/htba-announces-the-entry-of-miura-partners-as-the-majority-shareholder-in-its-new-phase-of-growth/

24 Sep 2024
// NBR
https://nutraceuticalbusinessreview.com/healthtech-bioactives-polyphenol-vitamin-b12-cphi-milan

14 May 2024
// PRESS RELEASE
https://htba.com/healthtech-bioactives-and-abolis-biotechnologies-partner-for-sustainable-high-value-polyphenol-production/#:~:text=Enhancing%20polyphenol%20production,for%20these%20in%2Ddemand%20ingredients.
Inspections and registrations
Country : Spain
City/Region : Beniel (Murcia)
Audit Date : 2025-03-26
Audit Type : On-Site
 FULL SCREEN VIEW Click here to open all results in a new tab [this preview display 10 results]
FULL SCREEN VIEW Click here to open all results in a new tab [this preview display 10 results]ABOUT THIS PAGE
HealthTech BioActives is a supplier offers 13 products (APIs, Excipients or Intermediates).
Find a price of Diosmin bulk with DMF, CEP offered by HealthTech BioActives
Find a price of Diosmetin bulk with DMF offered by HealthTech BioActives
Find a price of Methylcobalamin bulk with JDMF offered by HealthTech BioActives
Find a price of Cobamamide bulk offered by HealthTech BioActives
Find a price of Hesperidin bulk offered by HealthTech BioActives
Find a price of Hesperidin Methylchalcone bulk offered by HealthTech BioActives
Find a price of Hydroxocobalamin bulk offered by HealthTech BioActives
Find a price of Hydroxocobalamin Acetate bulk offered by HealthTech BioActives
Find a price of Hydroxocobalamin Hydrochloride bulk offered by HealthTech BioActives
Find a price of Neohesperidin bulk offered by HealthTech BioActives
Find a price of Neohesperidin Dihydrochalcone bulk offered by HealthTech BioActives
Find a price of Olive bulk offered by HealthTech BioActives
Find a price of Quercetin bulk offered by HealthTech BioActives