05 Jul 2024
// PRESS RELEASE
29 Jun 2024
// PRESS RELEASE
28 Jun 2024
// PRESS RELEASE
Latest Content by PharmaCompass
 KEY PRODUCTS
KEY PRODUCTS
Porton Pharma Solutions: Value creation via innovation & discovery through technical platforms.
About
CPhI India 2024CPhI India 2024
Industry Trade Show
Not Confirmed
26-28 November, 2024
Saudi International Ex...Saudi International Expo
Industry Trade Show
Not Confirmed
28-30 October, 2024
SupplySide West 2024SupplySide West 2024
Industry Trade Show
Not Confirmed
28 October-01 November, 2024
CONTACT DETAILS





Events
Webinars & Exhibitions
CPhI India 2024CPhI India 2024
Industry Trade Show
Not Confirmed
26-28 November, 2024
Saudi International Ex...Saudi International Expo
Industry Trade Show
Not Confirmed
28-30 October, 2024
SupplySide West 2024SupplySide West 2024
Industry Trade Show
Not Confirmed
28 October-01 November, 2024
CORPORATE CONTENT #SupplierSpotlight
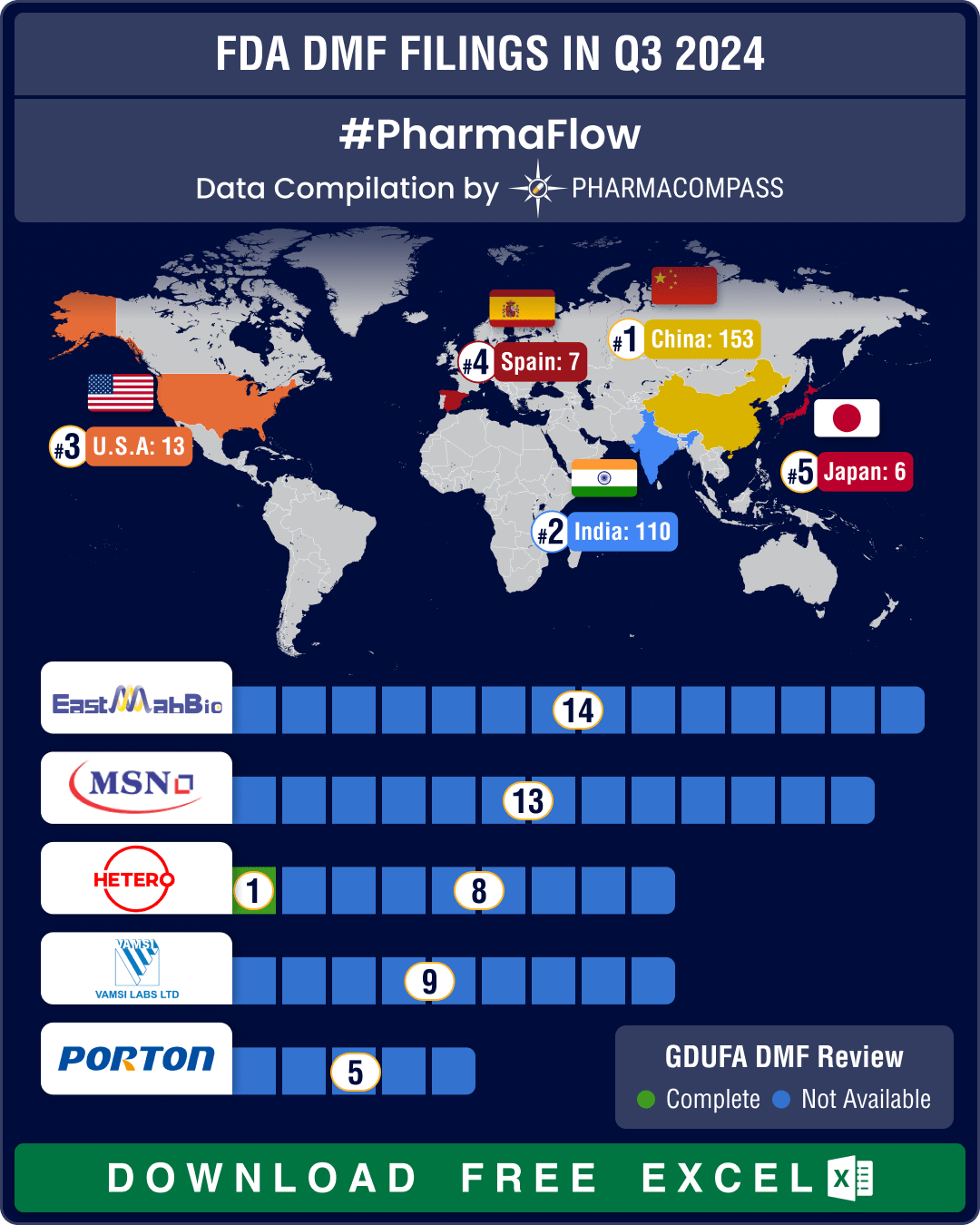
https://www.pharmacompass.com/radio-compass-blog/dmf-filings-hit-all-time-high-in-q3-2024-china-tops-list-with-58-increase-in-type-ii-submissions
https://www.pharmacompass.com/radio-compass-blog/cdmo-activity-tracker-novo-s-parent-buys-catalent-for-us-16-5-bn-fujifilm-merck-kgaa-axplora-lonza-expand-capabilities

05 Jul 2024
// PRESS RELEASE

29 Jun 2024
// PRESS RELEASE

28 Jun 2024
// PRESS RELEASE

06 Jun 2024
// PRESS RELEASE

23 May 2024
// PRESS RELEASE

16 May 2024
// PRESS RELEASE
 Click Us!
Click Us! 
GDUFA
DMF Review : N/A
Rev. Date :
Pay. Date :
DMF Number : 26541
Submission : 2012-10-03
Status : Active
Type : II

Certificate Number : R1-CEP 2012-386 - Rev 00
Issue Date : 2019-03-11
Type : Chemical
Substance Number : 1452
Status : Valid

NDC Package Code : 75877-0002
Start Marketing Date : 2012-02-27
End Marketing Date : 2024-12-31
Dosage Form (Strength) : POWDER (1kg/kg)
Marketing Category : BULK INGREDIENT

GDUFA
DMF Review : N/A
Rev. Date :
Pay. Date :
DMF Number : 39029
Submission : 2023-11-02
Status : Active
Type : II

GDUFA
DMF Review : N/A
Rev. Date :
Pay. Date :
DMF Number : 39028
Submission : 2023-10-16
Status : Active
Type : II

GDUFA
DMF Review : N/A
Rev. Date :
Pay. Date :
DMF Number : 33798
Submission : 2019-04-22
Status : Active
Type : II

NDC Package Code : 75877-0007
Start Marketing Date : 2019-03-26
End Marketing Date : 2024-12-31
Dosage Form (Strength) : POWDER (1kg/kg)
Marketing Category : BULK INGREDIENT

GDUFA
DMF Review : N/A
Rev. Date :
Pay. Date :
DMF Number : 38186
Submission : 2023-03-17
Status : Active
Type : II

GDUFA
DMF Review : Reviewed
Rev. Date : 2024-09-25
Pay. Date : 2024-08-28
DMF Number : 39012
Submission : 2023-11-10
Status : Active
Type : II

GDUFA
DMF Review : N/A
Rev. Date :
Pay. Date :
DMF Number : 15413
Submission : 2001-05-04
Status : Active
Type : II

NDC Package Code : 75877-0005
Start Marketing Date : 2017-11-28
End Marketing Date : 2024-12-31
Dosage Form (Strength) : POWDER (1kg/kg)
Marketing Category : BULK INGREDIENT

GDUFA
DMF Review : N/A
Rev. Date :
Pay. Date :
DMF Number : 38020
Submission : 2023-02-10
Status : Active
Type : II

NDC Package Code : 75877-0002
Start Marketing Date : 2012-02-27
End Marketing Date : 2024-12-31
Dosage Form (Strength) : POWDER (1kg/kg)
Marketing Category : BULK INGREDIENT

GDUFA
DMF Review : N/A
Rev. Date :
Pay. Date :
DMF Number : 37070
Submission : 2022-05-23
Status : Active
Type : II

GDUFA
DMF Review : N/A
Rev. Date :
Pay. Date :
DMF Number : 35714
Submission : 2021-05-19
Status : Active
Type : II
 FULL SCREEN VIEW Click here to open all results in a new tab [this preview display 10 results]
FULL SCREEN VIEW Click here to open all results in a new tab [this preview display 10 results]
GDUFA
DMF Review : N/A
Rev. Date :
Pay. Date :
DMF Number : 40093
Submission : 2024-06-13
Status : Active
Type : II
GDUFA
DMF Review : N/A
Rev. Date :
Pay. Date :
DMF Number : 39029
Submission : 2023-11-02
Status : Active
Type : II
GDUFA
DMF Review : N/A
Rev. Date :
Pay. Date :
DMF Number : 40455
Submission : 2024-09-05
Status : Active
Type : II
GDUFA
DMF Review : N/A
Rev. Date :
Pay. Date :
DMF Number : 39703
Submission : 2024-03-20
Status : Active
Type : II
GDUFA
DMF Review : N/A
Rev. Date :
Pay. Date :
DMF Number : 39028
Submission : 2023-10-16
Status : Active
Type : II
GDUFA
DMF Review : N/A
Rev. Date :
Pay. Date :
DMF Number : 33798
Submission : 2019-04-22
Status : Active
Type : II
GDUFA
DMF Review : N/A
Rev. Date :
Pay. Date :
DMF Number : 38186
Submission : 2023-03-17
Status : Active
Type : II
GDUFA
DMF Review : N/A
Rev. Date :
Pay. Date :
DMF Number : 35714
Submission : 2021-05-19
Status : Active
Type : II
GDUFA
DMF Review : Complete
Rev. Date : 2024-09-25
Pay. Date : 2024-08-28
DMF Number : 39012
Submission : 2023-11-10
Status : Active
Type : II
GDUFA
DMF Review : N/A
Rev. Date :
Pay. Date :
DMF Number : 15413
Submission : 2001-05-04
Status : Active
Type : II
 FULL SCREEN VIEW Click here to open all results in a new tab [this preview display 10 results]
FULL SCREEN VIEW Click here to open all results in a new tab [this preview display 10 results]Certificate Numbers : R1-CEP 2012-386 - Rev 00
Status : Valid
Issue Date : 2019-03-11
Type : Chemical
Substance Number : 1452
 FULL SCREEN VIEW Click here to open all results in a new tab [this preview display 10 results]
FULL SCREEN VIEW Click here to open all results in a new tab [this preview display 10 results]About the Company : Porton Pharma Solutions Ltd. was founded in 2005, & the company's stock was successfully listed in Shenzhen Stock Exchange in 2014. Our R&D, manufacturing & operation facilities are located in C...
About the Company : Porton Pharma Solutions Ltd. was founded in 2005, & the company's stock was successfully listed in Shenzhen Stock Exchange in 2014. Our R&D, manufacturing & operation facilities are located in C...
About the Company : Porton Pharma Solutions Ltd. was founded in 2005, & the company's stock was successfully listed in Shenzhen Stock Exchange in 2014. Our R&D, manufacturing & operation facilities are located in C...
About the Company : Porton Pharma Solutions Ltd. was founded in 2005, & the company's stock was successfully listed in Shenzhen Stock Exchange in 2014. Our R&D, manufacturing & operation facilities are located in C...
About the Company : Porton Pharma Solutions Ltd. was founded in 2005, & the company's stock was successfully listed in Shenzhen Stock Exchange in 2014. Our R&D, manufacturing & operation facilities are located in C...
About the Company : Porton Pharma Solutions Ltd. was founded in 2005, & the company's stock was successfully listed in Shenzhen Stock Exchange in 2014. Our R&D, manufacturing & operation facilities are located in C...
About the Company : Porton Pharma Solutions Ltd. was founded in 2005, & the company's stock was successfully listed in Shenzhen Stock Exchange in 2014. Our R&D, manufacturing & operation facilities are located in C...
About the Company : Porton Pharma Solutions Ltd. was founded in 2005, & the company's stock was successfully listed in Shenzhen Stock Exchange in 2014. Our R&D, manufacturing & operation facilities are located in C...
About the Company : Porton Pharma Solutions Ltd. was founded in 2005, & the company's stock was successfully listed in Shenzhen Stock Exchange in 2014. Our R&D, manufacturing & operation facilities are located in C...
About the Company : Porton Pharma Solutions Ltd. was founded in 2005, & the company's stock was successfully listed in Shenzhen Stock Exchange in 2014. Our R&D, manufacturing & operation facilities are located in C...
 FULL SCREEN VIEW Click here to open all results in a new tab [this preview display 10 results]
FULL SCREEN VIEW Click here to open all results in a new tab [this preview display 10 results]CAS Number : 162537-11-3
End Use API : Atazanavir
About the Company : Porton Pharma Solutions Ltd. was founded in 2005, & the company's stock was successfully listed in Shenzhen Stock Exchan...
Methyl (3R)-3-(tert- butyldimethylsilyloxy)-5- oxo-6- tripheny...
CAS Number : 147118-35-2
End Use API : Rosuvastatin Calcium
About the Company : Porton Pharma Solutions Ltd. was founded in 2005, & the company's stock was successfully listed in Shenzhen Stock Exchan...
2-amino-N-(2,2,2-trifluoroethyl)acetamide hydrochloride
CAS Number : 1171331-39-7
End Use API : Fluralaner
About the Company : Porton Pharma Solutions Ltd. was founded in 2005, & the company's stock was successfully listed in Shenzhen Stock Exchan...
trans-2-(4-acetamidocyclohexyl)acetic acid
CAS Number : 2901-44-2
End Use API : Cariprazine Hydrochloride
About the Company : Porton Pharma Solutions Ltd. was founded in 2005, & the company's stock was successfully listed in Shenzhen Stock Exchan...
5-bromo-2-chloro-4'- ethoxydiphenylmethane
CAS Number : 461432-23-5
End Use API : Dapagliflozin
About the Company : Porton Pharma Solutions Ltd. was founded in 2005, & the company's stock was successfully listed in Shenzhen Stock Exchan...
(2R,3R,4R,5S,6S)-2-(acetoxymethyl)-6-(4- chloro-3-(4- ethoxybe...
CAS Number : 461432-25-7
End Use API : Dapagliflozin
About the Company : Porton Pharma Solutions Ltd. was founded in 2005, & the company's stock was successfully listed in Shenzhen Stock Exchan...
(3R,4S,5R,6R)-3,4,5-tris(triMethylsilyloxy)-6- ((triMethylsily...
CAS Number : 32384-65-9
End Use API : Empagliflozin
About the Company : Porton Pharma Solutions Ltd. was founded in 2005, & the company's stock was successfully listed in Shenzhen Stock Exchan...
CAS Number : 86087-23-2
End Use API : Empagliflozin
About the Company : Porton Pharma Solutions Ltd. was founded in 2005, & the company's stock was successfully listed in Shenzhen Stock Exchan...
CAS Number : 86087-23-2
End Use API : Amprenavir
About the Company : Porton Pharma Solutions Ltd. was founded in 2005, & the company's stock was successfully listed in Shenzhen Stock Exchan...
CAS Number : 86087-23-2
End Use API : Fosamprenavir
About the Company : Porton Pharma Solutions Ltd. was founded in 2005, & the company's stock was successfully listed in Shenzhen Stock Exchan...
 FULL SCREEN VIEW Click here to open all results in a new tab [this preview display 10 results]
FULL SCREEN VIEW Click here to open all results in a new tab [this preview display 10 results]Services
API Manufacturing
Pharma Service : API Manufacturing
Category : Small Molecules
Sub Category : Overview
Pharma Service : API Manufacturing
 FULL SCREEN VIEW Click here to open all results in a new tab [this preview display 10 results]
FULL SCREEN VIEW Click here to open all results in a new tab [this preview display 10 results]Pharma Service : API Manufacturing
Category : Protein / Peptide
Sub Category : Overview
Pharma Service : API Manufacturing
 FULL SCREEN VIEW Click here to open all results in a new tab [this preview display 10 results]
FULL SCREEN VIEW Click here to open all results in a new tab [this preview display 10 results]Drug Product Manufacturing
API & Drug Product Development
Inspections and registrations
ABOUT THIS PAGE
Porton Pharma Solutions is a supplier offers 32 products (APIs, Excipients or Intermediates).
Find a price of Nicotine bulk with DMF, CEP offered by Porton Pharma Solutions
Find a price of Alectinib Hydrochloride bulk with DMF offered by Porton Pharma Solutions
Find a price of Apalutamide bulk with DMF offered by Porton Pharma Solutions
Find a price of Baloxavir Marboxil bulk with DMF offered by Porton Pharma Solutions
Find a price of Canagliflozin bulk with DMF offered by Porton Pharma Solutions
Find a price of CAS 1421271-01-3 bulk with DMF offered by Porton Pharma Solutions
Find a price of Dapagliflozin bulk with DMF offered by Porton Pharma Solutions
Find a price of Darunavir Ethanolate bulk with DMF offered by Porton Pharma Solutions
Find a price of Dexrazoxane bulk with DMF offered by Porton Pharma Solutions
Find a price of Isavuconazonium Sulfate bulk with DMF offered by Porton Pharma Solutions
Find a price of Mefloquine bulk with DMF offered by Porton Pharma Solutions
Find a price of Nicotine bulk with DMF offered by Porton Pharma Solutions
Find a price of Phloroglucinol bulk with DMF offered by Porton Pharma Solutions
Find a price of Relugolix bulk with DMF offered by Porton Pharma Solutions
Find a price of Salmeterol Xinafoate bulk with DMF offered by Porton Pharma Solutions
Find a price of Sulfanilamide bulk with DMF offered by Porton Pharma Solutions
Find a price of Tiopronin bulk with DMF offered by Porton Pharma Solutions
Find a price of Vibegron bulk with DMF offered by Porton Pharma Solutions
Find a price of TRIMETHYLPHLOROGLUCINOL DRUG SUBSTANCE (NON-STERILE) bulk with DMF offered by Porton Pharma Solutions
Find a price of Abemaciclib bulk offered by Porton Pharma Solutions
Find a price of Cariprazine Hydrochloride bulk offered by Porton Pharma Solutions
Find a price of Crisaborole bulk offered by Porton Pharma Solutions
Find a price of Dolutegravir Sodium bulk offered by Porton Pharma Solutions
Find a price of Empagliflozin bulk offered by Porton Pharma Solutions
Find a price of Hydroxychloroquine Sulphate bulk offered by Porton Pharma Solutions
Find a price of Methotrexate bulk offered by Porton Pharma Solutions
Find a price of Niraparib Tosylate bulk offered by Porton Pharma Solutions
Find a price of Palbociclib bulk offered by Porton Pharma Solutions
Find a price of Rimegepant Sulfate bulk offered by Porton Pharma Solutions
Find a price of Ruxolitinib Phosphate bulk offered by Porton Pharma Solutions
Find a price of Trimethyl Phloroglucinol bulk offered by Porton Pharma Solutions
Find a price of Vonoprazan Fumarate bulk offered by Porton Pharma Solutions


LOOKING FOR A SUPPLIER?