31 Jan 2025
// PRESS RELEASE
30 Jan 2023
// PRESS RELEASE
14 Nov 2022
// PRESS RELEASE
Latest Content by PharmaCompass
 KEY PRODUCTS
KEY PRODUCTS KEY SERVICES
KEY SERVICES
Farmhispania Group, a leading European CDMO in HPAPI Technologies & High Potency Fermentation.
About
CPhI North America CPhI North America
Industry Trade Show
Not Confirmed
20-22 May, 2025
German Wound CongressGerman Wound Congress
Industry Trade Show
Not Confirmed
07 April-09 May, 2025
Industry Trade Show
Not Confirmed
08 April-11 May, 2025
CONTACT DETAILS






Events
Webinars & Exhibitions
CPhI North America CPhI North America
Industry Trade Show
Not Confirmed
20-22 May, 2025
German Wound CongressGerman Wound Congress
Industry Trade Show
Not Confirmed
07 April-09 May, 2025
Industry Trade Show
Not Confirmed
08 April-11 May, 2025
CORPORATE CONTENT #SupplierSpotlight
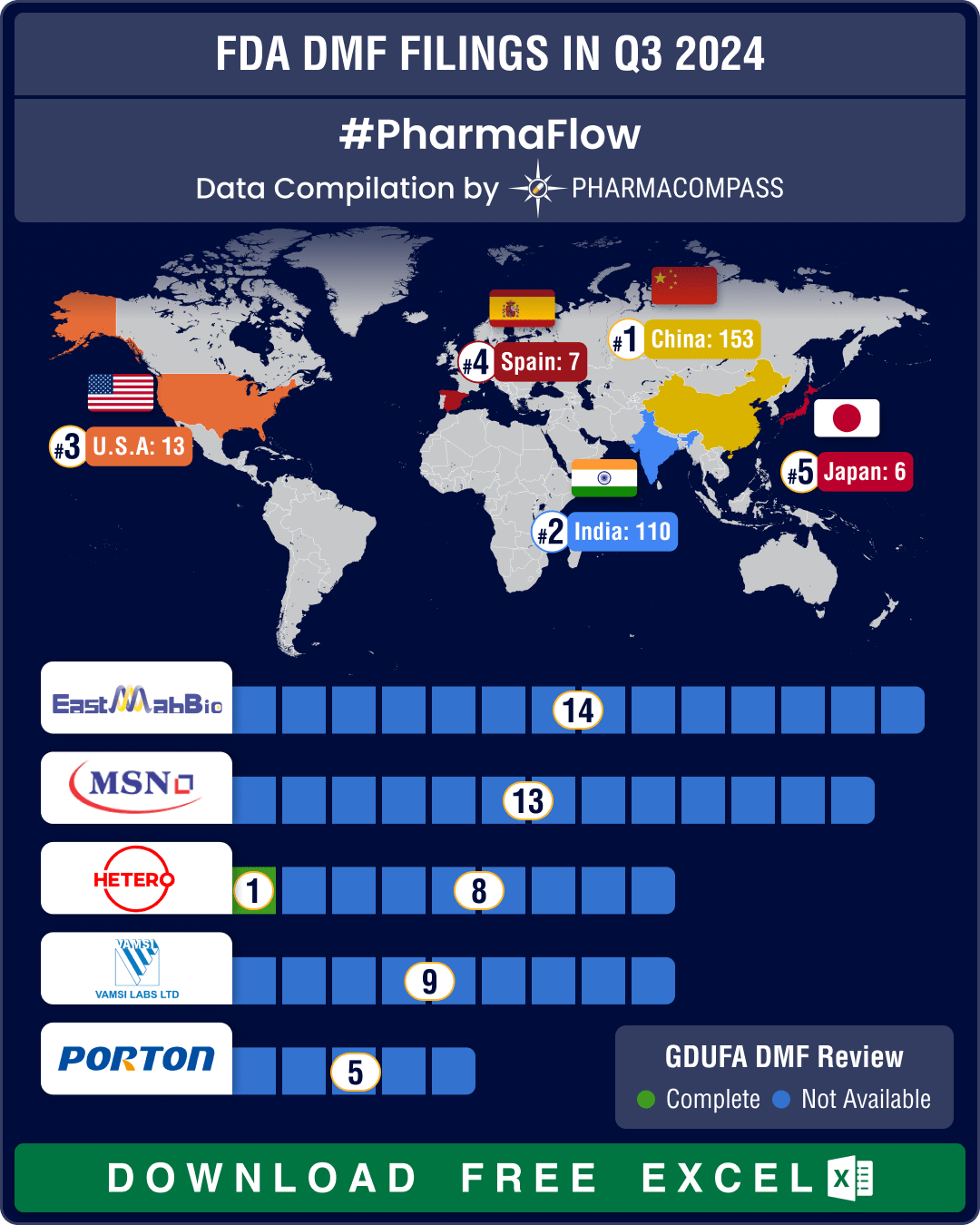
https://www.pharmacompass.com/radio-compass-blog/dmf-filings-hit-all-time-high-in-q3-2024-china-tops-list-with-58-increase-in-type-ii-submissions

31 Jan 2025
// PRESS RELEASE
https://www.farmhispaniagroup.com/nuevos-proyectos-implantados-mejora-de-la-eficiencia-de-los-procesos-de-generacion-de-agua-fria-2/

30 Jan 2023
// PRESS RELEASE
https://www.farmhispaniagroup.com/fhg-to-sponsor-felix-serratosa-conference/

14 Nov 2022
// PRESS RELEASE
https://www.farmhispaniagroup.com/fhgs-montmelo-site-successfully-inspected-by-anvisa/

02 Feb 2021
// PRESS RELEASE
https://www.farmhispaniagroup.com/framhispania-group-awarded-with-the-first-cep-for-everolimus/

12 Sep 2020
// PRESS RELEASE
https://www.farmhispaniagroup.com/new-multipurpose-manufacturing-building-at-the-montmelo-barcelona-site/

16 Mar 2020
// PRESS RELEASE
https://www.farmhispaniagroup.com/medicines-for-europe-statement-on-covid-19-coronavirus/
Services
API Manufacturing
API & Drug Product Development
Inspections and registrations
ABOUT THIS PAGE
Farmhispania is a supplier offers 45 products (APIs, Excipients or Intermediates).
Find a price of Metformin bulk with DMF, CEP, JDMF offered by Farmhispania
Find a price of Rocuronium Bromide bulk with DMF, CEP, JDMF offered by Farmhispania
Find a price of Atracurium Besylate bulk with DMF, CEP offered by Farmhispania
Find a price of Benazepril Hydrochloride bulk with DMF, CEP offered by Farmhispania
Find a price of Cisatracurium Besylate bulk with DMF, CEP offered by Farmhispania
Find a price of Enalapril Maleate bulk with DMF, CEP offered by Farmhispania
Find a price of Everolimus bulk with DMF, CEP offered by Farmhispania
Find a price of Gefitinib bulk with CEP, JDMF offered by Farmhispania
Find a price of Gemcitabine bulk with CEP, JDMF offered by Farmhispania
Find a price of Milrinone bulk with DMF, JDMF offered by Farmhispania
Find a price of Pemetrexed Disodium bulk with CEP, JDMF offered by Farmhispania
Find a price of Sugammadex Sodium bulk with DMF, JDMF offered by Farmhispania
Find a price of Ramipril bulk with CEP offered by Farmhispania
Find a price of Captopril bulk with CEP offered by Farmhispania
Find a price of Decitabine bulk with DMF offered by Farmhispania
Find a price of Deutetrabenazine bulk with DMF offered by Farmhispania
Find a price of Metformin bulk with DMF offered by Farmhispania
Find a price of Quinapril bulk with DMF offered by Farmhispania
Find a price of Rocuronium Bromide bulk with CEP offered by Farmhispania
Find a price of Tacrolimus bulk with DMF offered by Farmhispania
Find a price of GEMCITABINE HYDROCHLORIDE USP bulk with DMF offered by Farmhispania
Find a price of Atropine bulk offered by Farmhispania
Find a price of Atropine Sulfate bulk offered by Farmhispania
Find a price of Cannabidiol bulk offered by Farmhispania
Find a price of Delapril Hydrochloride bulk offered by Farmhispania
Find a price of Desloratadine bulk offered by Farmhispania
Find a price of Deucravacitinib bulk offered by Farmhispania
Find a price of Deutetrabenazine bulk offered by Farmhispania
Find a price of Loratadine bulk offered by Farmhispania
Find a price of Metformin bulk offered by Farmhispania
Find a price of Methotrexate bulk offered by Farmhispania
Find a price of Midostaurin bulk offered by Farmhispania
Find a price of Palonosetron bulk offered by Farmhispania
Find a price of Perindopril Arginine bulk offered by Farmhispania
Find a price of Perindopril Erbumine bulk offered by Farmhispania
Find a price of Pirfenidone bulk offered by Farmhispania
Find a price of Relugolix bulk offered by Farmhispania
Find a price of Sirolimus bulk offered by Farmhispania
Find a price of Staurosporine bulk offered by Farmhispania
Find a price of Tapentadol bulk offered by Farmhispania
Find a price of Tetrabenazine bulk offered by Farmhispania
Find a price of Trandolapril bulk offered by Farmhispania
Find a price of Valbenazine Tosylate bulk offered by Farmhispania
Find a price of Vecuronium Bromide bulk offered by Farmhispania
Find a price of Zavegepant HCl bulk offered by Farmhispania