20 Nov 2024
// PRESS RELEASE
07 Oct 2024
// PRESS RELEASE
08 Aug 2024
// PRESS RELEASE
Latest Content by PharmaCompass
 KEY PRODUCTS
KEY PRODUCTS KEY SERVICES
KEY SERVICES
Interquim comes from an international group of 50 companies active in the pharma, hospital, diagnostics, fine chemicals & feed sectors.
About
CPhI North America CPhI North America
Industry Trade Show
Not Confirmed
20-22 May, 2025
German Wound CongressGerman Wound Congress
Industry Trade Show
Not Confirmed
07 April-09 May, 2025
Industry Trade Show
Not Confirmed
08 April-11 May, 2025
CONTACT DETAILS




Events
Webinars & Exhibitions
CPhI North America CPhI North America
Industry Trade Show
Not Confirmed
20-22 May, 2025
German Wound CongressGerman Wound Congress
Industry Trade Show
Not Confirmed
07 April-09 May, 2025
Industry Trade Show
Not Confirmed
08 April-11 May, 2025
CORPORATE CONTENT #SupplierSpotlight
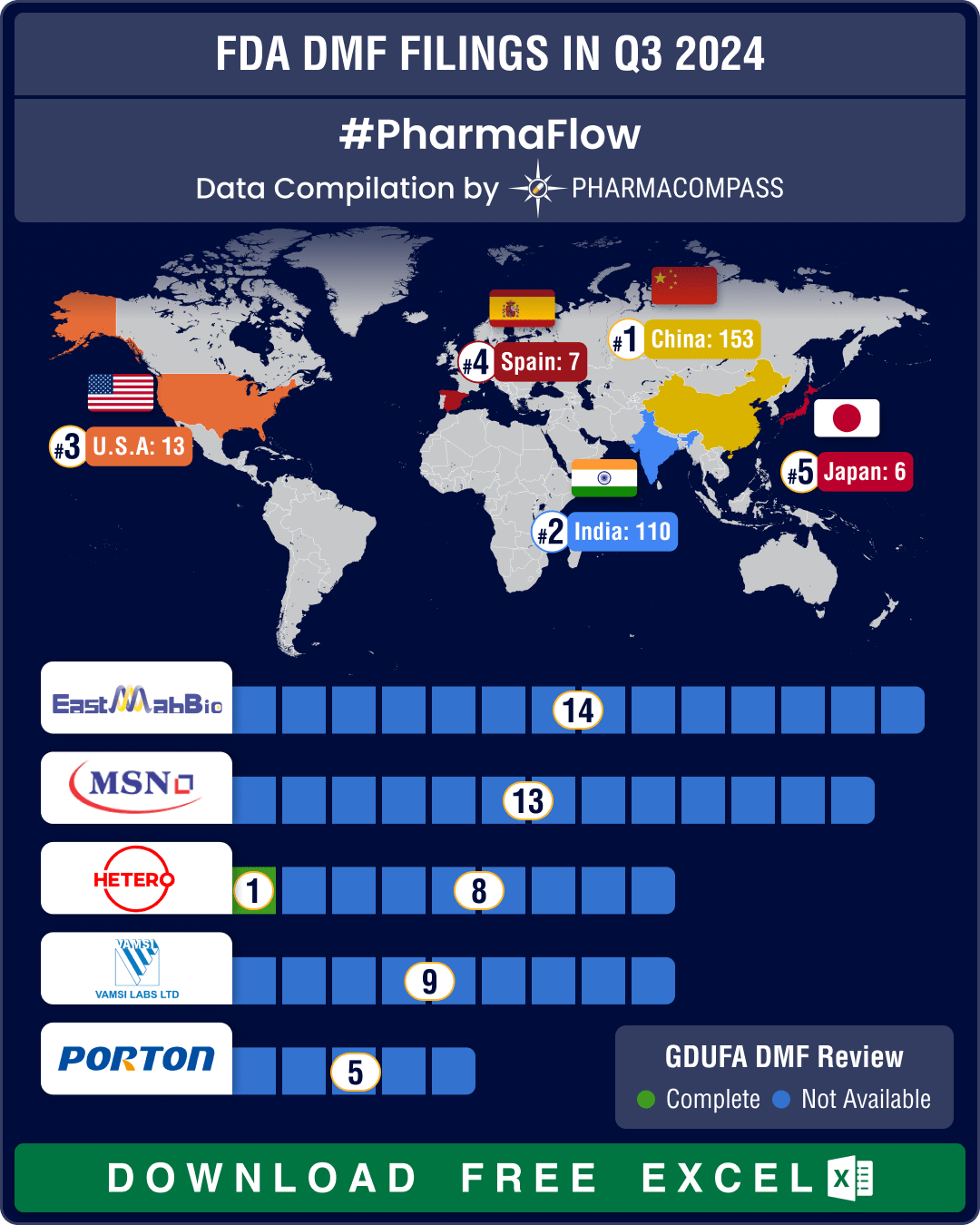
https://www.pharmacompass.com/radio-compass-blog/dmf-filings-hit-all-time-high-in-q3-2024-china-tops-list-with-58-increase-in-type-ii-submissions

20 Nov 2024
// PRESS RELEASE
https://www.ferrer.com/en/Ferrer_spearheads_two_European_studies_to_improve_the_diagnosis_and_treatment_of_pulmonary_hypertension_associated_with_interstitial_lung_disease

07 Oct 2024
// PRESS RELEASE
https://www.ferrer.com/sites/default/files/2024-07/Ferrer_Sustainability_Report_2023_web.pdf

08 Aug 2024
// PRESS RELEASE
https://www.ferrer.com/en/Ferrer-advances-research-Progressive-Supranuclear-Palsy-PSP

24 May 2024
// PRESS RELEASE
https://www.ferrer.com/en/Tyvaso-indications-idiopathic-pulmonary-fibrosis-progressive-pulmonary-fibrosis

21 Apr 2024
// PRESS RELEASE
https://www.ferrer.com/en/pacientes-hipertension-pulmonar-reivindica-sopoerte-psicologico

25 Mar 2024
// PRESS RELEASE
Services
API Manufacturing
API & Drug Product Development
Inspections and registrations
ABOUT THIS PAGE
Interquim SA is a supplier offers 49 products (APIs, Excipients or Intermediates).
Find a price of Rivastigmine bulk with DMF, CEP, JDMF offered by Interquim SA
Find a price of Celecoxib bulk with DMF, CEP offered by Interquim SA
Find a price of Olmesartan Medoxomil bulk with CEP, JDMF offered by Interquim SA
Find a price of Ozenoxacin bulk with DMF, JDMF offered by Interquim SA
Find a price of Rotigotine bulk with DMF, CEP offered by Interquim SA
Find a price of Sertaconazole Nitrate bulk with DMF, CEP offered by Interquim SA
Find a price of Solifenacin Succinate bulk with DMF, CEP offered by Interquim SA
Find a price of Tadalafil bulk with CEP, JDMF offered by Interquim SA
Find a price of Telmisartan bulk with CEP, JDMF offered by Interquim SA
Find a price of Tolterodine Tartrate bulk with DMF, CEP offered by Interquim SA
Find a price of Aripiprazole bulk with DMF offered by Interquim SA
Find a price of Aripiprazole Lauroxil bulk with DMF offered by Interquim SA
Find a price of Ciclopirox bulk with DMF offered by Interquim SA
Find a price of Ciclopirox Olamine bulk with CEP offered by Interquim SA
Find a price of Ferric Carboxymaltose bulk with DMF offered by Interquim SA
Find a price of Imiquimod bulk with DMF offered by Interquim SA
Find a price of Lifitegrast bulk with DMF offered by Interquim SA
Find a price of Paliperidone Palmitate bulk with DMF offered by Interquim SA
Find a price of Risperidone bulk with DMF offered by Interquim SA
Find a price of Rivastigmine Tartrate bulk with CEP offered by Interquim SA
Find a price of Roflumilast bulk with DMF offered by Interquim SA
Find a price of Acetylcholine Chloride bulk offered by Interquim SA
Find a price of Aripiprazole bulk offered by Interquim SA
Find a price of Citicoline Sodium bulk offered by Interquim SA
Find a price of Detomidine bulk offered by Interquim SA
Find a price of Dexmedetomidine Hydrochloride bulk offered by Interquim SA
Find a price of Edaravone bulk offered by Interquim SA
Find a price of Febuxostat bulk offered by Interquim SA
Find a price of Ferric Derisomaltose bulk offered by Interquim SA
Find a price of Fosfomycin Trometamol bulk offered by Interquim SA
Find a price of Hydroxocobalamin bulk offered by Interquim SA
Find a price of Lumateperone Tosylate bulk offered by Interquim SA
Find a price of Methylcobalamin bulk offered by Interquim SA
Find a price of Paliperidone bulk offered by Interquim SA
Find a price of Paliperidone Palmitate bulk offered by Interquim SA
Find a price of Pimavanserin Tartrate bulk offered by Interquim SA
Find a price of Pyrimethamine bulk offered by Interquim SA
Find a price of Rasagiline Tartrate bulk offered by Interquim SA
Find a price of Rifapentine bulk offered by Interquim SA
Find a price of Rivastigmine bulk offered by Interquim SA
Find a price of Roflumilast bulk offered by Interquim SA
Find a price of Selenious Acid bulk offered by Interquim SA
Find a price of Sertaconazole Nitrate bulk offered by Interquim SA
Find a price of Solifenacin Succinate bulk offered by Interquim SA
Find a price of Tapinarof bulk offered by Interquim SA
Find a price of Tolterodine Tartrate bulk offered by Interquim SA
Find a price of Torsemide bulk offered by Interquim SA
Find a price of Uridine Triphosphate Trisodium bulk offered by Interquim SA
Find a price of Vitamin B12 bulk offered by Interquim SA