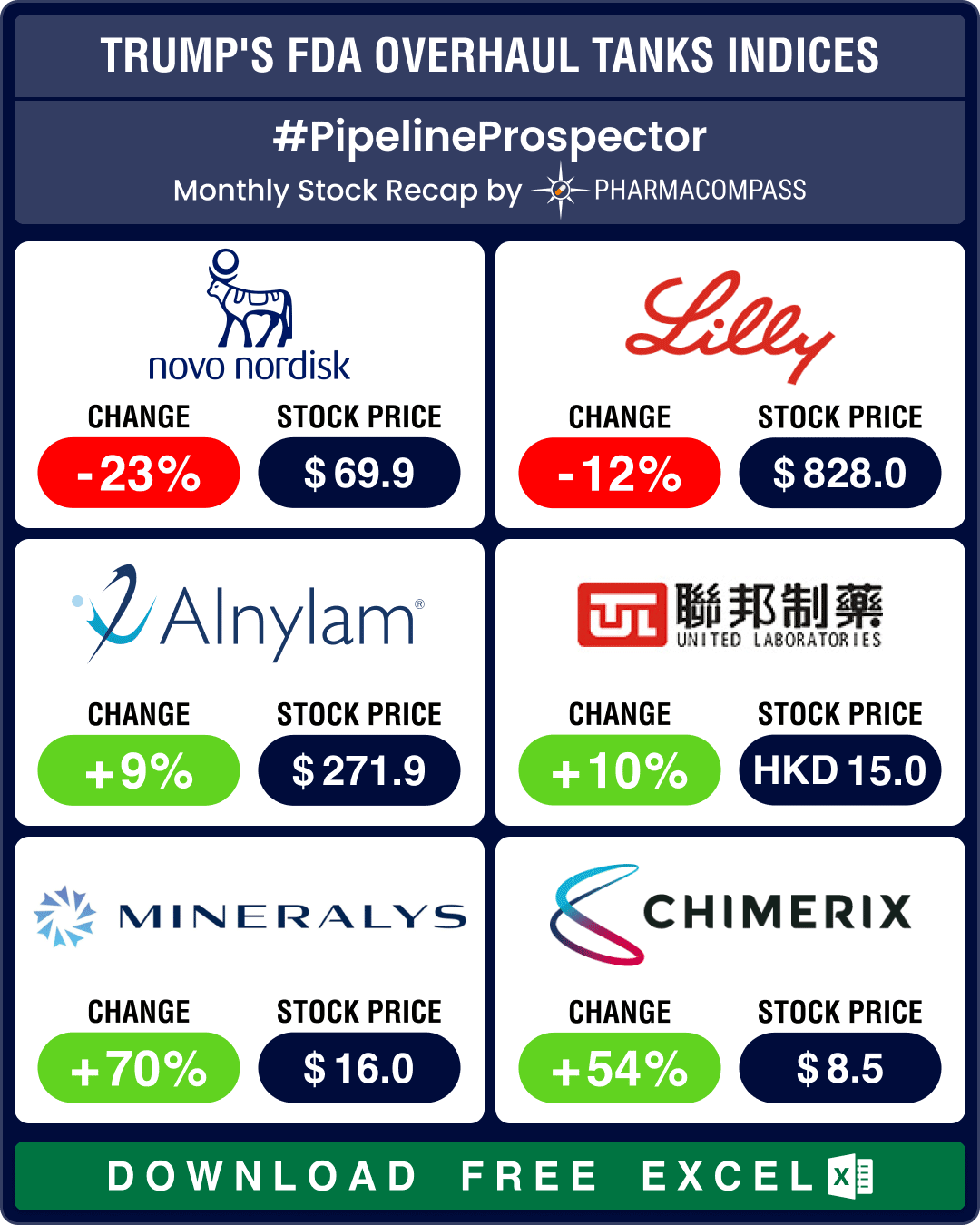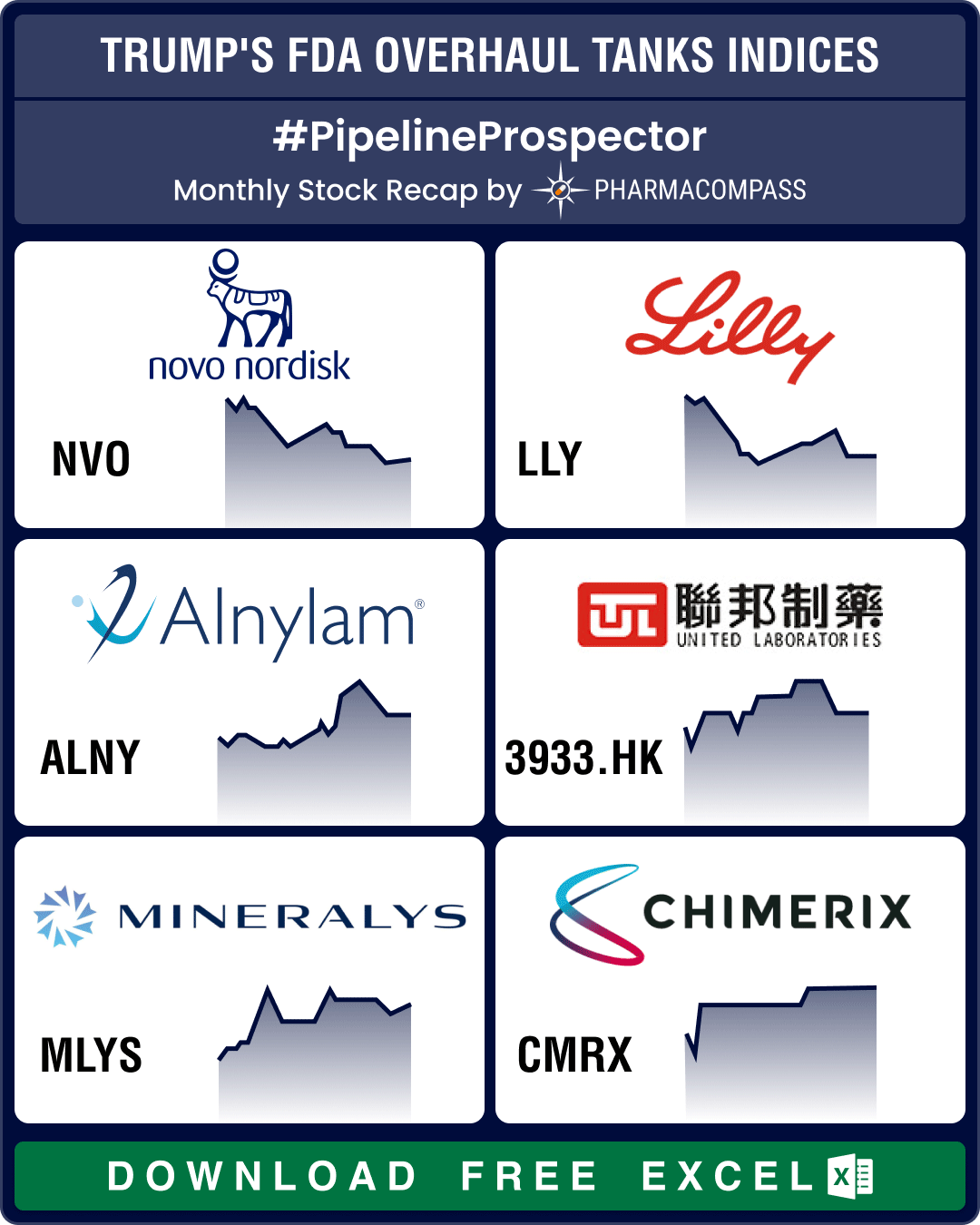
24 May 2023
// FDA
29 Jul 2020
// FDA
23 Aug 2016
// Gareth MacDonald+ IN PHARMATECHNOLOGIST
Latest Content by PharmaCompass

About
Industry Trade Show
Not Confirmed
09-11 April, 2025
Industry Trade Show
Attending
28-30 August, 2025
CPhI WW FrankfurtCPhI WW Frankfurt
Industry Trade Show
Booth #9.2B56
28-30 October, 2025
CONTACT DETAILS







Events
Webinars & Exhibitions
Industry Trade Show
Not Confirmed
09-11 April, 2025
Industry Trade Show
Attending
28-30 August, 2025
CPhI WW FrankfurtCPhI WW Frankfurt
Industry Trade Show
Booth #9.2B56
28-30 October, 2025

24 May 2023
// FDA
https://www.pharmacompass.com/pdf/news/enforcement-report-week-of-may-24-2023-89772.pdf

29 Jul 2020
// FDA
https://www.accessdata.fda.gov/scripts/cder/daf/index.cfm?event=overview.process&ApplNo=212721

23 Aug 2016
// Gareth MacDonald+ IN PHARMATECHNOLOGIST
http://www.in-pharmatechnologist.com/Regulatory-Safety/Sagent-recalls-second-lot-of-antibiotic-made-by-Astral-SteriTech-after-rust-found-in-vial
Services
Drug Product Manufacturing
API & Drug Product Development
Inspections and registrations
ABOUT THIS PAGE

 Astral SteriTech
Astral SteriTech