03 Apr 2025
// INDPHARMAPOST
27 Jan 2025
// PRESS FRELEASE
24 Jan 2025
// BUSINESS STD
Latest Content by PharmaCompass
 KEY PRODUCTS
KEY PRODUCTS
Delivering quality and niche Active Pharmaceutical Ingredients across the global from our USFDA-approved facility.
About
CPhI North America CPhI North America
Industry Trade Show
Not Confirmed
20-22 May, 2025
BIO International Conv...BIO International Convention
Industry Trade Show
Attending
16-19 June, 2025
Industry Trade Show
Attending
28-30 August, 2025
CONTACT DETAILS




Events
Webinars & Exhibitions
CPhI North America CPhI North America
Industry Trade Show
Not Confirmed
20-22 May, 2025
BIO International Conv...BIO International Convention
Industry Trade Show
Attending
16-19 June, 2025
Industry Trade Show
Attending
28-30 August, 2025
CORPORATE CONTENT #SupplierSpotlight
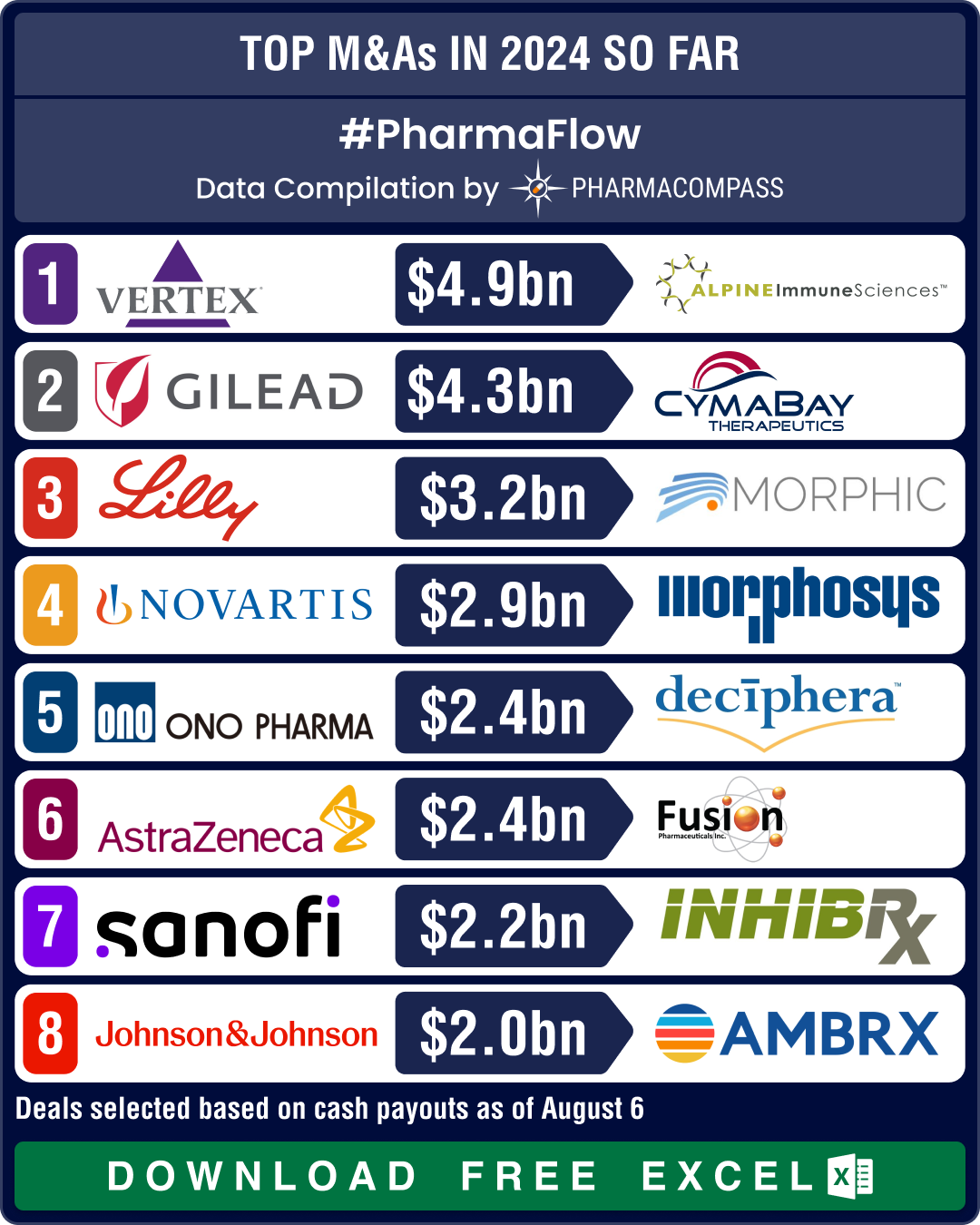
https://www.pharmacompass.com/radio-compass-blog/novartis-gsk-sanofi-bms-shell-out-over-us-10-bn-in-dealmaking-as-mid-size-deals-take-centerstage-in-2024

03 Apr 2025
// INDPHARMAPOST
https://www.indianpharmapost.com/news/mankind-unveils-healthoks-100-vegetarian-multivitamin-tablets-campaign-with-archana-puran-singh-16968

27 Jan 2025
// PRESS FRELEASE
https://www.mankindpharma.com/media/press-release/mankind-pharma-launches-45-day-healthcare-mission-at-mahakumbh-serving-thousands-of-pilgrims-with-free-health-check-ups

24 Jan 2025
// BUSINESS STD
https://www.business-standard.com/markets/capital-market-news/mankind-pharma-slides-after-q3-pat-slips-16-yoy-to-rs-380-cr-125012400521_1.html

24 Jan 2025
// BUSINESS STD
https://www.business-standard.com/markets/news/mankind-pharma-shares-crack-6-after-q3-profit-drops-16-know-more-125012400322_1.html

24 Jan 2025
// BUSINESS STD
https://www.business-standard.com/markets/capital-market-news/mankind-pharma-consolidated-net-profit-declines-16-20-in-the-december-2024-quarter-125012400174_1.html

23 Jan 2025
// BUSINESS STD
https://www.business-standard.com/companies/news/mankind-pharma-q3fy25-results-pat-falls-16-5-revenue-rises-24-125012301463_1.html
 Click Us!
Click Us! 
GDUFA
DMF Review : Reviewed
Rev. Date : 2022-01-12
Pay. Date : 2021-11-29
DMF Number : 34844
Submission : 2020-06-05
Status : Active
Type : II

Certificate Number : CEP 2022-262 - Rev 00
Issue Date : 2024-02-16
Type : Chemical
Substance Number : 2191
Status : Valid

GDUFA
DMF Review : Reviewed
Rev. Date : 2020-08-20
Pay. Date : 2020-07-21
DMF Number : 34935
Submission : 2020-07-30
Status : Active
Type : II

Certificate Number : R0-CEP 2020-310 - Rev 00
Issue Date : 2022-10-24
Type : Chemical
Substance Number : 889
Status : Valid

| Available Reg. Filing : ASMF |

GDUFA
DMF Review : Reviewed
Rev. Date : 2020-01-21
Pay. Date : 2019-11-14
DMF Number : 34190
Submission : 2019-11-22
Status : Active
Type : II

Certificate Number : CEP 2020-026 - Rev 01
Issue Date : 2024-02-22
Type : Chemical
Substance Number : 616
Status : Valid

| Available Reg. Filing : ASMF |

GDUFA
DMF Review : Reviewed
Rev. Date : 2020-03-30
Pay. Date : 2020-02-20
DMF Number : 34353
Submission : 2020-02-26
Status : Active
Type : II

Certificate Number : CEP 2020-080 - Rev 01
Issue Date : 2024-08-30
Type : Chemical
Substance Number : 1431
Status : Valid

| Available Reg. Filing : ASMF |

GDUFA
DMF Review : Reviewed
Rev. Date : 2019-04-26
Pay. Date : 2019-03-05
DMF Number : 33351
Submission : 2018-12-28
Status : Active
Type : II

Certificate Number : R0-CEP 2021-371 - Rev 00
Issue Date : 2021-12-10
Type : Chemical
Substance Number : 101
Status : Valid

| Available Reg. Filing : ASMF |

GDUFA
DMF Review : Reviewed
Rev. Date : 2019-04-26
Pay. Date : 2019-03-05
DMF Number : 33523
Submission : 2019-02-23
Status : Active
Type : II

Certificate Number : CEP 2019-092 - Rev 02
Issue Date : 2023-10-12
Type : Chemical
Substance Number : 101
Status : Valid

| Available Reg. Filing : ASMF |

GDUFA
DMF Review : Reviewed
Rev. Date : 2020-12-31
Pay. Date : 2020-12-07
DMF Number : 35357
Submission : 2020-12-07
Status : Active
Type : II

Certificate Number : R0-CEP 2021-073 - Rev 00
Issue Date : 2022-11-14
Type : Chemical
Substance Number : 2296
Status : Valid

| Available Reg. Filing : ASMF |

GDUFA
DMF Review : Reviewed
Rev. Date : 2021-06-16
Pay. Date : 2021-03-24
DMF Number : 35635
Submission : 2021-03-05
Status : Active
Type : II

Certificate Number : R0-CEP 2021-104 - Rev 00
Issue Date : 2023-08-30
Type : Chemical
Substance Number : 568
Status : Valid

| Available Reg. Filing : ASMF |

GDUFA
DMF Review : Reviewed
Rev. Date : 2022-07-11
Pay. Date : 2022-06-07
DMF Number : 36493
Submission : 2021-12-22
Status : Active
Type : II

Certificate Number : R0-CEP 2022-060 - Rev 00
Issue Date : 2023-06-26
Type : Chemical
Substance Number : 2631
Status : Valid

GDUFA
DMF Review : Reviewed
Rev. Date : 2022-06-09
Pay. Date : 2022-05-27
DMF Number : 36711
Submission : 2022-02-01
Status : Active
Type : II

Certificate Number : R0-CEP 2022-067 - Rev 00
Issue Date : 2023-07-06
Type : Chemical
Substance Number : 3087
Status : Valid
 FULL SCREEN VIEW Click here to open all results in a new tab [this preview display 10 results]
FULL SCREEN VIEW Click here to open all results in a new tab [this preview display 10 results]
GDUFA
DMF Review : Complete
Rev. Date : 2022-01-12
Pay. Date : 2021-11-29
DMF Number : 34844
Submission : 2020-06-05
Status : Active
Type : II
GDUFA
DMF Review : N/A
Rev. Date :
Pay. Date :
DMF Number : 36365
Submission : 2021-09-30
Status : Active
Type : II
GDUFA
DMF Review : Complete
Rev. Date : 2022-06-21
Pay. Date : 2022-06-01
DMF Number : 37011
Submission : 2022-04-27
Status : Active
Type : II
GDUFA
DMF Review : N/A
Rev. Date :
Pay. Date :
DMF Number : 39328
Submission : 2024-05-01
Status : Active
Type : II
GDUFA
DMF Review : Complete
Rev. Date : 2020-08-20
Pay. Date : 2020-07-21
DMF Number : 34935
Submission : 2020-07-30
Status : Active
Type : II
GDUFA
DMF Review : N/A
Rev. Date :
Pay. Date :
DMF Number : 37540
Submission : 2023-02-01
Status : Active
Type : II
GDUFA
DMF Review : Complete
Rev. Date : 2020-03-30
Pay. Date : 2020-02-20
DMF Number : 34353
Submission : 2020-02-26
Status : Active
Type : II
GDUFA
DMF Review : Complete
Rev. Date : 2020-01-21
Pay. Date : 2019-11-14
DMF Number : 34190
Submission : 2019-11-22
Status : Active
Type : II
GDUFA
DMF Review : Complete
Rev. Date : 2025-01-16
Pay. Date : 2025-01-06
DMF Number : 40408
Submission : 2024-11-18
Status : Active
Type : II
GDUFA
DMF Review : Complete
Rev. Date : 2019-04-26
Pay. Date : 2019-03-05
DMF Number : 33523
Submission : 2019-02-23
Status : Active
Type : II
 FULL SCREEN VIEW Click here to open all results in a new tab [this preview display 10 results]
FULL SCREEN VIEW Click here to open all results in a new tab [this preview display 10 results]Inspections and registrations
ABOUT THIS PAGE
Mankind Pharma is a supplier offers 45 products (APIs, Excipients or Intermediates).
Find a price of Atorvastatin bulk with DMF, CEP offered by Mankind Pharma
Find a price of Clomipramine Hydrochloride bulk with DMF, CEP offered by Mankind Pharma
Find a price of Haloperidol bulk with DMF, CEP offered by Mankind Pharma
Find a price of Haloperidol Decanoate bulk with DMF, CEP offered by Mankind Pharma
Find a price of Nitrofurantoin bulk with DMF, CEP offered by Mankind Pharma
Find a price of Pantoprazole Sodium bulk with DMF, CEP offered by Mankind Pharma
Find a price of Propranolol Hydrochloride bulk with DMF, CEP offered by Mankind Pharma
Find a price of Rosuvastatin Calcium bulk with DMF, CEP offered by Mankind Pharma
Find a price of Ticagrelor bulk with DMF, CEP offered by Mankind Pharma
Find a price of Atorvastatin bulk with DMF offered by Mankind Pharma
Find a price of Baclofen bulk with DMF offered by Mankind Pharma
Find a price of Cilnidipine bulk with DMF offered by Mankind Pharma
Find a price of Clozapine bulk with DMF offered by Mankind Pharma
Find a price of Midodrine bulk with DMF offered by Mankind Pharma
Find a price of Pantoprazole Sodium bulk with DMF offered by Mankind Pharma
Find a price of Ranolazine bulk with DMF offered by Mankind Pharma
Find a price of Revefenacin bulk with DMF offered by Mankind Pharma
Find a price of Rocuronium Bromide bulk with DMF offered by Mankind Pharma
Find a price of Sacubitril Sodium bulk with DMF offered by Mankind Pharma
Find a price of Sacubitril-Valsartan bulk with DMF offered by Mankind Pharma
Find a price of Sitagliptin Phosphate bulk with DMF offered by Mankind Pharma
Find a price of Succinylcholine Chloride bulk with DMF offered by Mankind Pharma
Find a price of Sugammadex Sodium bulk with DMF offered by Mankind Pharma
Find a price of Telmisartan bulk with DMF offered by Mankind Pharma
Find a price of Valsartan bulk with DMF offered by Mankind Pharma
Find a price of Brivaracetam bulk offered by Mankind Pharma
Find a price of Dydrogesterone bulk offered by Mankind Pharma
Find a price of Empagliflozin bulk offered by Mankind Pharma
Find a price of Ensifentrine bulk offered by Mankind Pharma
Find a price of Etoricoxib bulk offered by Mankind Pharma
Find a price of Fezolinetant bulk offered by Mankind Pharma
Find a price of Lasmiditan bulk offered by Mankind Pharma
Find a price of Latanoprostene Bunod bulk offered by Mankind Pharma
Find a price of Lifitegrast bulk offered by Mankind Pharma
Find a price of Netarsudil bulk offered by Mankind Pharma
Find a price of Netarsudil Mesylate bulk offered by Mankind Pharma
Find a price of Obeticholic Acid bulk offered by Mankind Pharma
Find a price of Perfluorohexyloctane bulk offered by Mankind Pharma
Find a price of Resmetirom bulk offered by Mankind Pharma
Find a price of Semaglutide bulk offered by Mankind Pharma
Find a price of Suzetrigine bulk offered by Mankind Pharma
Find a price of Upadacitinib bulk offered by Mankind Pharma
Find a price of Ursodeoxycholic Acid bulk offered by Mankind Pharma
Find a price of Xanomeline bulk offered by Mankind Pharma
Find a price of Sacubitril·Valsartan sodium chloride hydrate bulk offered by Mankind Pharma