18 Mar 2025
// PRESS RELEASE
12 Feb 2025
// FDA
31 Jan 2025
// PRESS RELEASE
Latest Content by PharmaCompass
 KEY PRODUCTS
KEY PRODUCTS
Strides Pharma Science: Pioneering IP-driven formulations for niche finished dosage forms.
About
CPhI North America CPhI North America
Industry Trade Show
Not Confirmed
20-22 May, 2025
CPhI WW FrankfurtCPhI WW Frankfurt
Industry Trade Show
Booth # 9.1F6 & 9.1G41
28-30 October, 2025
Industry Trade Show
Attending
25-27 November, 2025
CONTACT DETAILS





Events
Webinars & Exhibitions
CPhI North America CPhI North America
Industry Trade Show
Not Confirmed
20-22 May, 2025
CPhI WW FrankfurtCPhI WW Frankfurt
Industry Trade Show
Booth # 9.1F6 & 9.1G41
28-30 October, 2025
Industry Trade Show
Attending
25-27 November, 2025
CORPORATE CONTENT #SupplierSpotlight
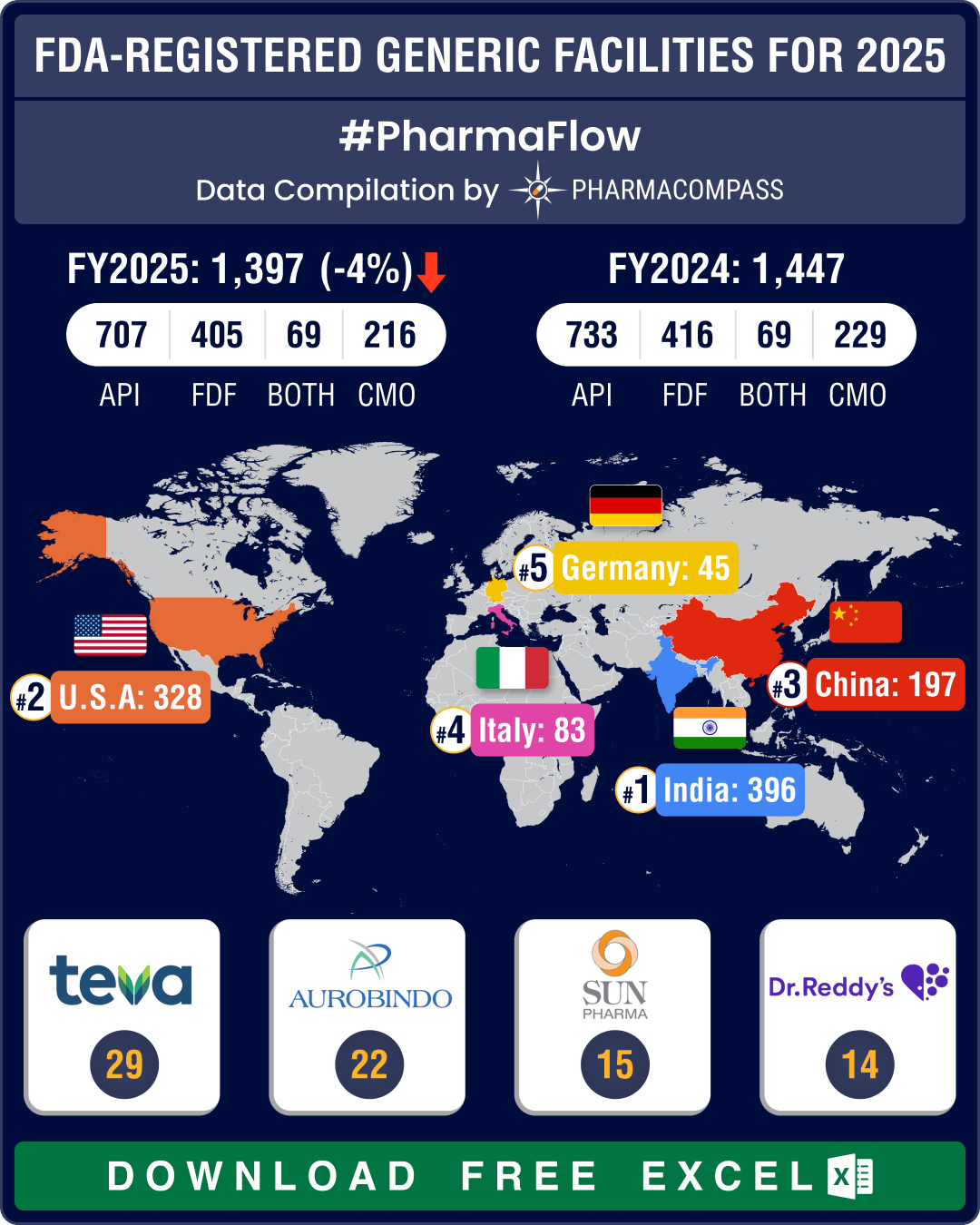
https://www.pharmacompass.com/radio-compass-blog/chinese-fda-registered-generic-facilities-gain-steam-india-maintains-lead-with-396-facilities

18 Mar 2025
// PRESS RELEASE
Chttps://strides.com/pdf/pressrelease/2025/StridesSEIntimation_signed_14_Mar_2025.pdf

12 Feb 2025
// FDA
https://www.pharmacompass.com/pdf/news/enforcement-report-week-of-february-12-2025-94329.pdf

31 Jan 2025
// PRESS RELEASE
https://strides.com/pdf/pressrelease/2025/newspaper_ad_31_jan_2025.pdf

23 Jan 2025
// PRESS RELEASE
https://strides.com/pdf/pressrelease/2025/StridesSEIntimation_22Jan_2025.pdf

22 Jan 2025
// PRESS RELEASE
https://strides.com/pdf/pressrelease/2025/StridesSEIntimation_22Jan_2025.pdf

20 Jan 2025
// EXPRESSPHARMA
https://www.expresspharma.in/strides-gains-usfda-approval-for-acetaminophen-and-ibuprofen-tablets-125-mg-250-mg/
Inspections and registrations
District Decision : Voluntary Action Indicated
Inspection End Date : 2023-02-24
City : Puducherry
State :
Country/Area : IN
Zip :
District :
Center :
Project Area : Drug Quality Assurance
District Decision : Voluntary Action Indicated
Inspection End Date : 2023-02-24
District Decision : Voluntary Action Indicated
Inspection End Date : 2022-12-09
City : Bangalore
State :
Country/Area : IN
Zip :
District :
Center :
Project Area : Drug Quality Assurance
District Decision : Voluntary Action Indicated
Inspection End Date : 2022-12-09
District Decision : No Action Indicated
Inspection End Date : 2022-08-08
City : Singapore
State :
Country/Area : SG
Zip :
District :
Center :
Project Area : Drug Quality Assurance
District Decision : No Action Indicated
Inspection End Date : 2022-08-08
District Decision : Voluntary Action Indicated
Inspection End Date : 2022-02-08
City : Chestnut Ridge
State :
Country/Area : US
Zip :
District :
Center :
Project Area : Drug Quality Assurance
District Decision : Voluntary Action Indicated
Inspection End Date : 2022-02-08
District Decision : No Action Indicated
Inspection End Date : 2020-03-05
City : Bangalore
State :
Country/Area : IN
Zip :
District :
Center :
Project Area : Drug Quality Assurance
District Decision : No Action Indicated
Inspection End Date : 2020-03-05
District Decision : Voluntary Action Indicated
Inspection End Date : 2020-01-17
City : Bangalore
State :
Country/Area : IN
Zip :
District :
Center :
Project Area : Drug Quality Assurance
District Decision : Voluntary Action Indicated
Inspection End Date : 2020-01-17
District Decision : No Action Indicated
Inspection End Date : 2019-08-23
City : Chestnut Ridge
State :
Country/Area : US
Zip :
District :
Center :
Project Area : Bioresearch Monitoring
District Decision : No Action Indicated
Inspection End Date : 2019-08-23
District Decision : Voluntary Action Indicated
Inspection End Date : 2019-06-05
City : Chestnut Ridge
State :
Country/Area : US
Zip :
District :
Center :
Project Area : Drug Quality Assurance
District Decision : Voluntary Action Indicated
Inspection End Date : 2019-06-05
District Decision : Voluntary Action Indicated
Inspection End Date : 2019-05-24
City : Bangalore
State :
Country/Area : IN
Zip :
District :
Center :
Project Area : Drug Quality Assurance
District Decision : Voluntary Action Indicated
Inspection End Date : 2019-05-24
District Decision : No Action Indicated
Inspection End Date : 2018-11-02
City : Bangalore
State :
Country/Area : IN
Zip :
District :
Center :
Project Area : Drug Quality Assurance
District Decision : No Action Indicated
Inspection End Date : 2018-11-02
 FULL SCREEN VIEW Click here to open all results in a new tab [this preview display 10 results]
FULL SCREEN VIEW Click here to open all results in a new tab [this preview display 10 results]ABOUT THIS PAGE