
Synopsis
Synopsis
0
JDMF
0
EU WC
0
KDMF
0
NDC API
0
VMF
0
Listed Suppliers
0
FDF
0
FDA Orange Book

0
Europe

0
Canada

0
Australia

0
South Africa

DRUG PRODUCT COMPOSITIONS
0
EDQM
0
USP
0
JP
0
Others
0
US Patents
0
US Exclusivities
0
Health Canada Patents
0
Data Compilation #PharmaFlow
0
Stock Recap #PipelineProspector
0
Weekly News Recap #Phispers
0
News #PharmaBuzz
US Medicaid
NA
Annual Reports
NA
Finished Drug Prices
NA

1. Alveograf
2. Calcitite
3. Calcium Hydroxyapatite
4. Durapatite
5. Hydroxyapatite, Calcium
6. Hydroxylapatite
7. Interpore 200
8. Interpore 500
9. Interpore-200
10. Interpore-500
11. Interpore200
12. Interpore500
13. Osprovit
14. Ossein Hydroxyapatite Compound
15. Ossein-hydroxyapatite Compound
16. Ossopan
17. Osteogen
18. Periograf
1. Durapatite
2. 12167-74-7
3. Hydroxylapatite
4. Alveograf
5. Periograf
6. Calcium Hydroxyapatite
7. Radiesse
8. Tribasic Calcium Phosphate
9. Durapatite [usan]
10. Calcium Hydroxide Phosphate
11. Win 40350
12. Calcium Hydroxylapatite
13. Calcium Hydroxide Tris(phosphate)
14. Pentacalcium;hydroxide;triphosphate
15. Hydroxylapatite, Ceramic
16. Ossopan
17. Calcium Phosphate, Tribasic [nf]
18. 91d9gv0z28
19. Durapatite (usan)
20. Mfcd00010904
21. Win-40350
22. Apatite, Hydroxy
23. Supertite 10
24. Calcium Phosphate, Tribasic (nf)
25. Pentacalcium Hydroxide Triphosphate
26. Hydroxylapatite (ca5(oh)(po4)3)
27. Calcium Orthophosphate, Basic
28. Hsdb 5804
29. Dekacalcium-dihydrat-hexa(phosphat)
30. Calcium Tribasic Phosphate
31. Einecs 215-145-7
32. Pentacalcium Monohydroxyorthophosphate
33. Pentacalcium Hydroxide Tris(orthophosphate)
34. Unii-91d9gv0z28
35. Hydroxyl Apatite
36. Alveograf (tn)
37. Decacalcium Hexaphosphate Dihydroxide
38. Einecs 235-330-6
39. Durapatite [mi]
40. Microcrystalline Hydroxyapatite Concentrate
41. Hydroxyapatite Nanopowder
42. Hydroxyapatite Micronpowder
43. Hydroxylapatite, Fast Flow
44. Calcarea Phosphorica
45. Ec 235-330-6
46. Hydroxyapatite Nanoparticles
47. Durapatite [who-dd]
48. Calcium Hydroxide Phosphate (ca10(oh)2(po4)6)
49. Hydroxyapatite [inci]
50. Hydroxyapatite [mart.]
51. Chembl2218916
52. Calcium Phosphate,tribasic
53. Calcium Orthophosphates Nanopowder
54. Dtxsid50872537
55. Hydroxylapatite, (ca 35-40%)
56. Calcarea Phosphorica [hpus]
57. Calcium Phosphate [who-ip]
58. Calcium Phosphate (tribasic)
59. Calcium Hydroxyapatite [inci]
60. Hydroxyapatite Nanoparticles Dispersion
61. Calcii Phosphas [who-ip Latin]
62. Tribasic Calcium Phosphate [ii]
63. Calcium Phosphate,tribasic [vandf]
64. Db-041626
65. Tribasic Calcium Phosphate [usp-rs]
66. Tribasic Calcium Phosphate [who-ip]
67. Ft-0630391
68. D03303
69. D03922
70. Calcium Phosphate Tribasic 34 To 40% Calcium Basis
71. J-004582
72. J-005837
73. Q27271399
74. Hydroxyapatite Nanoparticles, 5-10% (w/v) Aqueous Colloidal Dispersion, 20-50nm Particles
75. Pentacalcium Hydroxide Tris(orthophosphate), With A Fluorine Content Of Less Than 0,005 % By Weight On The Dry Anhydrous Product
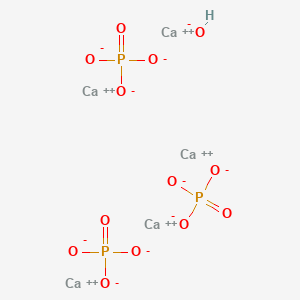
| Molecular Weight | 502.3 g/mol |
|---|---|
| Molecular Formula | Ca5HO13P3 |
| Hydrogen Bond Donor Count | 1 |
| Hydrogen Bond Acceptor Count | 13 |
| Rotatable Bond Count | 0 |
| Exact Mass | 501.675955 g/mol |
| Monoisotopic Mass | 501.675955 g/mol |
| Topological Polar Surface Area | 260 Ų |
| Heavy Atom Count | 21 |
| Formal Charge | 0 |
| Complexity | 36.8 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 0 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 9 |
EXPT USE: WOMEN WITH PRIMARY BILIARY CIRRHOSIS MALABSORB CALCIUM, PHOSPHATE & VITAMIN D, & DEVELOP ACCELERATED CORTICAL BONE THINNING. HYDROXYAPATITE WAS ASSESSED FOR ITS VALUE IN THE TREATMENT OF CORTICAL BONE THINNING IN PRIMARY BILIARY CIRRHOSIS. CORTICAL BONE LOSS OCCURRED IN THE CONTROL GROUP. THE HYDROXYAPATITE GROUP SHOWED A SIGNIFICANT GAIN IN CORTICAL BONE THICKNESS.
PMID:6287835 EPSTEIN O ET AL; AM J CLIN NUTR 36 (3): 426 (1982)
Biocompatible Materials
Synthetic or natural materials, other than DRUGS, that are used to replace or repair any body TISSUES or bodily function. (See all compounds classified as Biocompatible Materials.)
 Click Us!
Click Us! 
GDUFA
DMF Review : N/A
Rev. Date :
Pay. Date :
DMF Number : 10881
Submission : 1994-04-22
Status : Active
Type : II


GDUFA
DMF Review : N/A
Rev. Date :
Pay. Date :
DMF Number : 10914
Submission : 1994-04-22
Status : Active
Type : II

USDMF, CEP/COS, JDMF, EU-WC, NDC, KDMF, VMF, Others
USDMF, CEP/COS, JDMF, EU-WC, NDC, KDMF, VMF, Others
GDUFA
DMF Review : N/A
Rev. Date :
Pay. Date :
DMF Number : 9283
Submission : 1991-08-13
Status : Inactive
Type : II

Portfolio PDF
Product Web Link
Virtual Booth
Digital Content
Website
Corporate PDF
USDMF, CEP/COS, JDMF, EU-WC, NDC, KDMF, VMF, Others
USDMF, CEP/COS, JDMF, EU-WC, NDC, KDMF, VMF, Others
GDUFA
DMF Review : N/A
Rev. Date :
Pay. Date :
DMF Number : 10238
Submission : 1993-04-14
Status : Inactive
Type : II

Portfolio PDF
Product Web Link
Virtual Booth
Digital Content
Website
Corporate PDF
USDMF, CEP/COS, JDMF, EU-WC, NDC, KDMF, VMF, Others
USDMF, CEP/COS, JDMF, EU-WC, NDC, KDMF, VMF, Others
GDUFA
DMF Review : N/A
Rev. Date :
Pay. Date :
DMF Number : 11149
Submission : 1994-10-19
Status : Inactive
Type : II

Portfolio PDF
Product Web Link
Virtual Booth
Digital Content
Website
Corporate PDF
GDUFA
DMF Review : N/A
Rev. Date :
Pay. Date :
DMF Number : 11212
Submission : 1994-11-21
Status : Inactive
Type : II

GDUFA
DMF Review : N/A
Rev. Date :
Pay. Date :
DMF Number : 19628
Submission : 2006-07-20
Status : Inactive
Type : II

GDUFA
DMF Review : N/A
Rev. Date :
Pay. Date :
DMF Number : 9438
Submission : 1991-11-15
Status : Inactive
Type : II

Certificate Number : R0-CEP 2001-132 - Rev 00
Issue Date : 2001-12-20
Type : TSE
Substance Number :
Status : Withdrawn by Holder


Certificate Number : R1-CEP 2001-133 - Rev 01
Issue Date : 2014-01-10
Type : TSE
Substance Number :
Status : Valid

 FULL SCREEN VIEW Click here to open all results in a new tab [this preview display 10 results]
FULL SCREEN VIEW Click here to open all results in a new tab [this preview display 10 results]
Market Place

ABOUT THIS PAGE


LOOKING FOR A SUPPLIER?