
1. Ara C
2. Ara-c
3. Arabinofuranosylcytosine
4. Arabinoside, Cytosine
5. Arabinosylcytosine
6. Aracytidine
7. Aracytine
8. Beta Ara C
9. Beta-ara C
10. Cytarabine
11. Cytarabine Hydrochloride
12. Cytonal
13. Cytosar U
14. Cytosar-u
15. Cytosine Arabinoside
1. Cytarabin
2. 4-amino-1-pentofuranosylpyrimidin-2(1h)-one
3. .beta.-arabinosylcytosine
4. .beta.-cytosine Arabinoside
5. .beta.-d-arabinosylcytosine
6. Cytosine-.beta.-arabinoside
7. Arabinofuranosylcytosine
8. Aracytidine
9. Cytarabinoside
10. Spongocytidine
11. 1.beta.-d-arabinosylcytosine
12. Alexan
13. Mls003389283
14. Cytosine, .beta.-d-arabinoside
15. 1.beta.-arabinofuranasylcytosine
16. Arabinosylcytosine
17. Cytosine Arabinose
18. Nci-c04728
19. 1.beta.-d-arabinofuranosylcytosine
20. 4-amino-1-[3,4-dihydroxy-5-(hydroxymethyl)oxolan-2-yl]pyrimidin-2-one
21. Cytosine, 1-.beta.-d-arabinosyl-
22. Cytosine-.beta.-d-arabinofuranoside
23. 1-.beta.-d-arabinofaranosylcytosine
24. Cylocide
25. Nsc 63878
26. Cytosine, 1-.beta.-d-arabinofuranosyl-
27. 1-arabinofuranosylcytosine
28. 4-amino-1-[3,4-dihydroxy-5-(hydroxymethyl)tetrahydrofuran-2-yl]pyrimidin-2(1h)-one
29. Cytarabina
30. Arabitin
31. Arafcyt
32. Erpalfa
33. 688007-26-3
34. Iretin
35. 1-.beta.-d-arabinofuranosyl-4-amino-2(1h)pyrimidinone
36. Cytosine-beta-d-arabinofuranoside
37. Cytosine Beta-d-riboside;cytosine-1-beta-d-ribofuranoside
38. Cytosine Beta-d-arabinofuranoside;cytosine Arabinoside;ara-c
39. Mls002702869
40. 4-amino-1-(3,4-dihydroxy-5-(hydroxymethyl)tetrahydrofuran-2-yl)pyrimidin-2(1h)-one
41. Cytosine Beta-d-arabinofuranoside Hydrochloride;cytosine Arabinoside Hydrochloride;ara-c Hydrochloride
42. Smr001549941
43. 1.beta.-ribofuranosylcytosine
44. 1.beta.-d-ribofuranosylcytosine
45. 4-amino-1-arabinofuranosyl-2-oxo-1,2-dihydropyrimidine
46. 1-.beta.-d-arabinofuranosylcytosine
47. U 19920
48. Mfcd00006545
49. Mfcd00066487
50. Nsc287459
51. .beta.-d-ribofuranoside, Cytosine-1
52. Cytarabine, Cytarabine (cytosine 1-b-d-arabinofuranoside), Cytidine
53. Cytosine, 1-.beta.-d-ribofuranosyl-
54. Ac-1075
55. Nsc 287459
56. Ac 1075
57. Chembl78
58. 4-amino-1.beta.-d-ribofuranosyl-2(1h)-pyrimidinone
59. Chemdiv1_027335
60. Molmap_000003
61. 2(1h)-pyrimidinone, 4-amino-1-.beta.-d-ribofuranosyl-
62. Oprea1_593929
63. Oprea1_858801
64. Schembl149563
65. Cytosine .beta.-d-arabinoside
66. 1-(b-d-xylofuranosyl)cytosine
67. Hms664k11
68. Dtxsid80859078
69. 1-.beta.-arabinofuranosylcytosine
70. 1-.beta.-d-ribofuranosylcytosine
71. Hms3371g12
72. Hms3393k19
73. Hms3428o03
74. Hms3655p13
75. Albb-021997
76. Chx-3311
77. Nsc20258
78. Bbl028243
79. Nsc249004
80. Stk391124
81. Akos001590380
82. Akos017259253
83. Cytosine-1-.beta.-d-arabinofuranoside
84. Nsc-249004
85. Smp2_000209
86. Ncgc00015258-02
87. Ncgc00015258-03
88. Ncgc00015258-04
89. Ncgc00015258-05
90. Ncgc00094638-01
91. Ncgc00094638-02
92. Ncgc00094638-03
93. Ncgc00094638-04
94. As-12090
95. Nci60_012951
96. Sy004943
97. Sy005417
98. Db-124991
99. U-19920 A
100. Ft-0624314
101. Ft-0624315
102. Ft-0774114
103. Wln: T6nvnj Dz A-bt5otj Cq Dq E1q
104. Ab01273973-01
105. L001298
106. Sr-01000597029
107. Sr-01000597029-1
108. 2(1h)-pyrimidinone, 4-amino-1.beta.-d-ribofuranosyl-
109. 4-amino-1-.beta.-d-arabinofuranosyl-2(1h)-pyrimidinon
110. 4-amino-1-.beta.-d-arabinofuranosyl-2(1h)-pyrimidinone
111. 2(1h)-pyrimidinone, 4-amino-1.beta.-d-arabinofuranosyl-
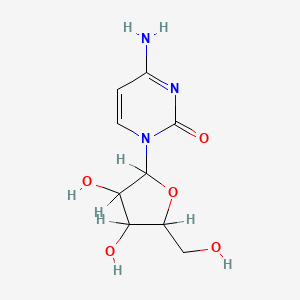
| Molecular Weight | 243.22 g/mol |
|---|---|
| Molecular Formula | C9H13N3O5 |
| XLogP3 | -2.1 |
| Hydrogen Bond Donor Count | 4 |
| Hydrogen Bond Acceptor Count | 5 |
| Rotatable Bond Count | 2 |
| Exact Mass | 243.08552052 g/mol |
| Monoisotopic Mass | 243.08552052 g/mol |
| Topological Polar Surface Area | 129 Ų |
| Heavy Atom Count | 17 |
| Formal Charge | 0 |
| Complexity | 383 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 0 |
| Undefined Atom Stereocenter Count | 4 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 1 |
Antiviral Agents
Agents used in the prophylaxis or therapy of VIRUS DISEASES. Some of the ways they may act include preventing viral replication by inhibiting viral DNA polymerase; binding to specific cell-surface receptors and inhibiting viral penetration or uncoating; inhibiting viral protein synthesis; or blocking late stages of virus assembly. (See all compounds classified as Antiviral Agents.)
Antimetabolites, Antineoplastic
Antimetabolites that are useful in cancer chemotherapy. (See all compounds classified as Antimetabolites, Antineoplastic.)
Immunosuppressive Agents
Agents that suppress immune function by one of several mechanisms of action. Classical cytotoxic immunosuppressants act by inhibiting DNA synthesis. Others may act through activation of T-CELLS or by inhibiting the activation of HELPER CELLS. While immunosuppression has been brought about in the past primarily to prevent rejection of transplanted organs, new applications involving mediation of the effects of INTERLEUKINS and other CYTOKINES are emerging. (See all compounds classified as Immunosuppressive Agents.)
BUILDING BLOCK

