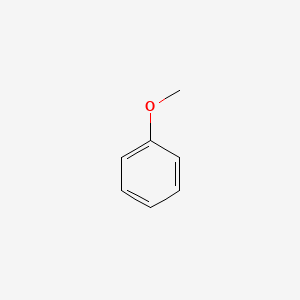

1. Anisol
2. Methoxybenzene
3. Methyl Phenyl Ether
4. Phenyl Methyl Ether
1. Methoxybenzene
2. 100-66-3
3. Methyl Phenyl Ether
4. Benzene, Methoxy-
5. Anisol
6. Phenyl Methyl Ether
7. Phenoxymethane
8. Anizol
9. Phenol Methyl Ether
10. Ether, Methyl Phenyl
11. Methoxy-benzene
12. 4-methoxybenzene
13. Hsdb 44
14. Fema No. 2097
15. Methylphenyl Ether
16. Nsc 7920
17. Ether, Methyl Phenyl-
18. B3w693gazh
19. Chebi:16579
20. Nsc-7920
21. Mfcd00008354
22. Benzene, Methoxy
23. Fema Number 2097
24. Einecs 202-876-1
25. Un2222
26. Unii-b3w693gazh
27. Anisole-
28. Ai3-00042
29. 4-methoxy Benzene
30. Anethole,(s)
31. Methyl Phenyl-ether
32. Anisole, 8ci
33. Anisole Methoxybenzene
34. Methoxy-benzeneanisole
35. Anisole-[13c]
36. Anisole [fhfi]
37. Anisole [hsdb]
38. Anisole [fcc]
39. Anisole [mi]
40. Methoxy-benzene (anisol)
41. Anisole [usp-rs]
42. Bmse010217
43. Ec 202-876-1
44. Schembl1205
45. Wln: 1or
46. Anisole, Analytical Standard
47. Methoxylated Aromatic Compound
48. Anisole, Anhydrous, 99.7%
49. Schembl497674
50. Aqualine™ Standard 1.1
51. A Methoxylated Aromatic Compound
52. Chembl278024
53. Dtxsid4041608
54. Schembl12015260
55. Anisole, Reagentplus(r), 99%
56. Nsc7920
57. Chebi:192244
58. Methoxy-benzene (anisol)
59. Anisole, >=99%, Fcc, Fg
60. Zinc897131
61. Amy38503
62. Bdbm50386177
63. Stl263485
64. Anisole 1000 Microg/ml In Methanol
65. Akos000120161
66. Zinc329788065
67. Zinc329788108
68. Ccg-266043
69. Un 2222
70. Anisole [un2222] [flammable Liquid]
71. Ls-13275
72. Db-003588
73. A0492
74. Ft-0628309
75. Ft-0652964
76. Ft-0662229
77. A14924
78. C01403
79. M03556
80. Q312244
81. J-000194
82. F1908-0172
83. Anisole, United States Pharmacopeia (usp) Reference Standard
84. Anisole, Pharmaceutical Secondary Standard; Certified Reference Material
| Molecular Weight | 108.14 g/mol |
|---|---|
| Molecular Formula | C7H8O |
| XLogP3 | 2.1 |
| Hydrogen Bond Donor Count | 0 |
| Hydrogen Bond Acceptor Count | 1 |
| Rotatable Bond Count | 1 |
| Exact Mass | 108.057514874 g/mol |
| Monoisotopic Mass | 108.057514874 g/mol |
| Topological Polar Surface Area | 9.2 Ų |
| Heavy Atom Count | 8 |
| Formal Charge | 0 |
| Complexity | 55.4 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 0 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 1 |
IN SITU PERFUSION IN RAT USED TO STUDY GASTROINTESTINAL ABSORPTION OF 4 FOOD ADDITIVES DERIVED FROM METHOXYBENZENE INCL ANISOLE. CMPD LARGELY WERE ABSORBED FROM DIGESTIVE TRACT BY PASSIVE DIFFUSION. ABSORPTION KINETICS VARY & ARE EXPLAINED BY DIFFERENCES IN LIPOSOLUBILITY.
PMID:1158324 FRITSCH P ET AL; FOOD COSMET TOXICOL 13 (3): 359 (1975)
ANISOLE YIELDS GUAIACOL, P-METHOXYPHENOL, AND PHENOL IN RABBITS. /FROM TABLE/
Goodwin, B.L. Handbook of Intermediary Metabolism of Aromatic Compounds. New York: Wiley, 1976., p. A-58
SEVERAL STRAINS OF ASPERGILLUS NIGER HYDROXYLATED ANISOLE TO GIVE O-HYDROXYANISOLE AS THE MAIN PRODUCT.
BOCKS SM; PHYTOCHEMISTRY 6 (6): 785 (1967)
... THE MAJOR METABOLITE OF ANISOLE ... /IS/ P-HYDROXYPHENYL METHYL ETHER, WHICH WAS EXCRETED UNCONJUGATED (2%) AND CONJUGATED WITH GLUCURONIC ACID (48%) AND SULFURIC ACID (29%).
Clayton, G. D. and F. E. Clayton (eds.). Patty's Industrial Hygiene and Toxicology: Volume 2A, 2B, 2C: Toxicology. 3rd ed. New York: John Wiley Sons, 1981-1982., p. 2521
... ADMINISTERING ANISOLE TO THE DOG CAUSED AN INCREASE IN THE EXCRETION OF ETHEREAL SULFATE.
Clayton, G. D. and F. E. Clayton (eds.). Patty's Industrial Hygiene and Toxicology: Volume 2A, 2B, 2C: Toxicology. 3rd ed. New York: John Wiley Sons, 1981-1982., p. 2521
For more Metabolism/Metabolites (Complete) data for ANISOLE (7 total), please visit the HSDB record page.
BUILDING BLOCK

