

1. Masflex
2. Miloxicam
3. Mobec
4. Mobic
5. Mobicox
6. Movalis
7. Movicox
8. Parocin
9. Reumoxicam
10. Uticox
1. 71125-38-7
2. Mobic
3. Metacam
4. Movalis
5. Movatec
6. Mobec
7. Mobicox
8. Meloxicam (mobic)
9. Uh-ac 62xx
10. Meloxivet
11. Coxicam
12. Melfax
13. Parocin
14. Meloxicamum [latin]
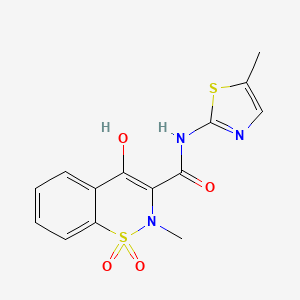
15. 4-hydroxy-2-methyl-n-(5-methylthiazol-2-yl)-2h-benzo[e][1,2]thiazine-3-carboxamide 1,1-dioxide
16. 4-hydroxy-2-methyl-n-(5-methyl-1,3-thiazol-2-yl)-2h-1,2-benzothiazine-3-carboxamide 1,1-dioxide
17. Meloxicamum
18. Meloxidolor
19. Melosus
20. Meloxoral
21. Rheumocam
22. Vivlodex
23. Revitacam
24. Acticam
25. Contacera
26. Emdocam
27. Flexicam
28. Inflacam
29. Loxicom
30. Melovem
31. Meloxidyl
32. Recocam
33. Unii-vg2qf83cgl
34. Uhac-62xx
35. Vg2qf83cgl
36. Chembl599
37. Uh-ac-62 Xx
38. 4-hydroxy-2-methyl-n-(5-methyl-2-thiazolyl)-2h-1,2-benzothiazine-3-carboxamide 1,1-dioxide
39. Mls000028587
40. Chebi:6741
41. N-1539
42. Ncgc00018248-04
43. Meloxicam 100 Microg/ml In Acetonitrile
44. Smr000058994
45. Dsstox_cid_803
46. N1539
47. 2h-1,2-benzothiazine-3-carboxamide, 4-hydroxy-2-methyl-n-(5-methyl-2-thiazolyl)-, 1,1-dioxide
48. 4-hydroxy-2-methyl-n-(5-methyl-2-thiazolyl)-2h-1,2-benzothiazine-3-carboxamide-1,1-dioxide
49. Dsstox_rid_75796
50. Dsstox_gsid_20803
51. 4-hydroxy-2-methyl-n-(5-methyl-1,3-thiazol-2-yl)-1,1-dioxo-2h-1$l^{6},2-benzothiazine-3-carboxamide
52. Revmoksikam
53. Coxflam
54. Melonex
55. Tenaron
56. (3e)-3-[hydroxy-[(5-methyl-1,3-thiazol-2-yl)amino]methylidene]-2-methyl-1,1-dioxo-1lambda6,2-benzothiazin-4-one
57. Cas-71125-38-7
58. Ccris 9139
59. Hsdb 7741
60. Meloxicam [usan:usp:inn:ban]
61. Meloxicam,(s)
62. 4-hydroxy-2-methyl-n-(5-methyl-1,3-thiazol-2-yl)-1,1-dioxo-1?^{6},2-benzothiazine-3-carboxamide
63. Mfcd00868752
64. Anjeso
65. Mobic (tn)
66. Opera_id_2
67. Qmiiz Odt
68. Spectrum_001633
69. Meloxicam [inn]
70. Meloxicam [jan]
71. Meloxicam [mi]
72. Meloxicam [hsdb]
73. Meloxicam [usan]
74. Spectrum2_000941
75. Spectrum3_000649
76. Spectrum4_000787
77. Spectrum5_001813
78. Meloxicam [vandf]
79. Meloxicam Impurity Standard
80. Meloxicam [mart.]
81. Meloxicam [usp-rs]
82. Meloxicam [who-dd]
83. Schembl3576
84. Meloxicam (jan/usp/inn)
85. Lopac0_000766
86. Schembl33369
87. Bspbio_002257
88. Kbiogr_001234
89. Kbioss_002113
90. Mls001304725
91. Mls001306413
92. Mls006011422
93. Bidd:gt0726
94. Schembl713100
95. Spectrum1504150
96. Spbio_000902
97. Meloxicam [green Book]
98. Gtpl7220
99. Meloxicam [orange Book]
100. Chembl1741042
101. Dtxsid1020803
102. Meloxicam [ep Monograph]
103. Schembl23589638
104. Kbio2_002113
105. Kbio2_004681
106. Kbio2_007249
107. Kbio3_001477
108. Eti-511
109. Meloxicam [usp Monograph]
110. Zeleris Component Meloxicam
111. Hms1922d19
112. Hms2089b18
113. Hms2096i19
114. Hms2234p07
115. Hms2236c09
116. Hms3259h06
117. Hms3372c13
118. Hms3372p18
119. Hms3655k06
120. Hms3713i19
121. Hms3744k09
122. Hms3884g08
123. Zynrelef Component Meloxicam
124. Albb-027268
125. Amy40407
126. Bcp11928
127. Hy-b0261
128. Tox21_111734
129. Tox21_113192
130. Tox21_201689
131. Tox21_302756
132. Bbl029076
133. Bdbm50056998
134. Ccg-39098
135. Hsci1_000045
136. S1734
137. Stk620505
138. Zinc13129998
139. Meloxicam - Cas 71125-38-7
140. Meloxicam Component Of Zeleris
141. Akos000279442
142. Akos026749959
143. Meloxicam Component Of Zynrelef
144. Tox21_111734_1
145. Zinc103620661
146. Ac-1325
147. Db00814
148. Ks-1084
149. Meloxicam [ema Epar Veterinary]
150. Nc00698
151. 2h-1,2-benzothiazine-3-carboxamide, 4-hydroxy-2-methyl-n-(5-methylthiazolyl)-, 1,1-dioxide
152. Smp2_000133
153. Ncgc00018248-01
154. Ncgc00018248-02
155. Ncgc00018248-03
156. Ncgc00018248-05
157. Ncgc00018248-06
158. Ncgc00018248-07
159. Ncgc00018248-08
160. Ncgc00018248-10
161. Ncgc00018248-23
162. Ncgc00018248-25
163. Ncgc00018248-26
164. Ncgc00022924-03
165. Ncgc00022924-04
166. Ncgc00256316-01
167. Ncgc00259238-01
168. Ncgc00263878-02
169. (e)-3-(hydroxy((5-methylthiazol-2-yl)amino)methylene)-2-methyl-2,3-dihydro-4h-benzo[e][1,2]thiazin-4-one 1,1-dioxide
170. 4-hydroxy-2-methyl-n-(5-methyl-1,3-thiazol-2-yl)-1,1-dioxo-1lambda6,2-benzothiazine-3-carboxamide
171. Bh164661
172. Smr000718800
173. Db-055490
174. Meloxicam 1.0 Mg/ml In Dimethyl Sulfoxide
175. Ft-0628193
176. M1959
177. Sw219562-1
178. En300-52507
179. C08169
180. D00969
181. F20356
182. Ab00383033-17
183. Ab00383033-18
184. Ab00383033_20
185. 125m387
186. A837087
187. An-668/13244001
188. Q414028
189. Sr-01000003132-10
190. F2173-0387
191. Z1695493323
192. 4-hydroxy-2-methyl-n-(5-methy-2-thiazolyl)-2h-1,2-benzothiazine-3-caboxamide-1,1-dioxide
193. (3z)-2-methyl-3-[[(5-methyl-1,3-thiazol-2-yl)amino]-oxidanyl-methylidene]-1,1-bis(oxidanylidene)-1$l^{6},2-benzothiazin-4-one
194. (3z)-3-[hydroxy-[(5-methyl-2-thiazolyl)amino]methylidene]-2-methyl-1,1-dioxo-1$l^{6},2-benzothiazin-4-one
195. 133687-22-6
196. 4-hydroxy-1,1-dioxo-1,2-dihydro-1lambda*6*-benzo[e][1,2]thiazine-3-carboxylic Acid (5-methyl-thiazol-2-yl)-amide
197. 4-hydroxy-2-methyl-1,1-dioxo-1,2-dihydro-1lambda*6*-benzo[e][1,2]thiazine-3-carboxylic Acid (5-methyl-thiazol-2-yl)-amide
198. 4-hydroxy-2-methyl-n-(5-methyl-1,3-thiazol-2-yl)-1,1-dioxo-1$l^{6},2-benzothiazine-3-carboxamide
199. 4-hydroxy-2-methyl-n-(5-methyl-1,3-thiazol-2-yl)-1,1-dioxo-2h-1lambda6,2-benzothiazine-3-carboxamide
200. 4-hydroxy-2-methyl-n-(5-methyl-2-thiazole)-2h-1,2-benzothiazine-3 -carboxamide 1,1-dioxide
201. 4-hydroxy-2-methyl-n-(5-methylthiazol-2-yl)-2h-benzo[e][1,2]thiazine-3-carboxamide1,1-dioxide
| Molecular Weight | 351.4 g/mol |
|---|---|
| Molecular Formula | C14H13N3O4S2 |
| XLogP3 | 3 |
| Hydrogen Bond Donor Count | 2 |
| Hydrogen Bond Acceptor Count | 7 |
| Rotatable Bond Count | 2 |
| Exact Mass | 351.03474825 g/mol |
| Monoisotopic Mass | 351.03474825 g/mol |
| Topological Polar Surface Area | 136 Ų |
| Heavy Atom Count | 23 |
| Formal Charge | 0 |
| Complexity | 628 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 0 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 1 |
| 1 of 3 | |
|---|---|
| Drug Name | MELOXICAM |
| Active Ingredient | MELOXICAM |
| Company | APOTEX INC (Application Number: A077882); AUROBINDO PHARMA (Application Number: A078008); BRECKENRIDGE PHARM (Application Number: A077920); CIPLA LTD (Application Number: A077929); DR REDDYS LABS INC (Application Number: A077931); GLENMARK GENERICS (Application Number: A077932); LUPIN PHARMS (Application Number: A077944); MYLAN (Application Number: A077923); PURACAP PHARM (Application Number: A077938); STRIDES PHARMA (Application Number: A077928); TARO (Application Number: A078102); TEVA PHARMS (Application Number: A077936); UNICHEM (Application Number: A077927); YUNG SHIN PHARM (Application Number: A077918); ZYDUS PHARMS USA (Application Number: A077921) |
| 2 of 3 | |
|---|---|
| Drug Name | VIVLODEX |
| Active Ingredient | MELOXICAM |
| Company | IROKO PHARMS LLC (Application Number: N207233. Patents: 9526734, 9649318, 9808468) |
| 3 of 3 | |
|---|---|
| Drug Name | MOBIC |
| Active Ingredient | MELOXICAM |
| Company | BOEHRINGER INGELHEIM (Application Number: N020938) |
Thiazines, Thiazoles; Isoenzymes/antagonists & inhibitors
National Library of Medicine's Medical Subject Headings online file (MeSH, 2009)
Meloxicam is indicated for relief of the signs and symptoms of osteoarthritis. Use the lowest effective dose for the shortest duration consistent with individual patient treatment goals. /Included in US product label/
US Natl Inst Health; DailyMed. Current Medication Information for Meloxicam (meloxicam) tablets (September 2007). Available from, as of June 29, 2009: https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?id=5437
Meloxicam is used for the management of the signs and symptoms of rheumatoid arthritis in adults. In the management of rheumatoid arthritis in adults, NSAIAs may be useful for initial symptomatic treatment; however, NSAIAs do not alter the course of the disease or prevent joint destruction. /Included in US product label/
American Society of Health System Pharmacists; AHFS Drug Information 2009. Bethesda, MD. (2009), p. 2161
Meloxicam is used for the management of the signs and symptoms of pauciarticular or polyarticular course juvenile rheumatoid arthritis in children 2 years of age or older. /NOT included in US product label/
American Society of Health System Pharmacists; AHFS Drug Information 2009. Bethesda, MD. (2009), p. 2161
For more Therapeutic Uses (Complete) data for Meloxicam (7 total), please visit the HSDB record page.
/BOXED WARNING/ WARNING: Cardiovascular Risk NSAIDs may cause an increased risk of serious cardiovascular thrombotic events, myocardial infarction, and stroke, which can be fatal. This risk may increase with duration of use. Patients with cardiovascular disease or risk factors for cardiovascular disease may be at greater risk. Meloxicam is contraindicated for the treatment of peri-operative pain in the setting of coronary artery bypass graft (CABG) surgery.
US Natl Inst Health; DailyMed. Current Medication Information for Meloxicam (meloxicam) tablets (Updated: May 2010). Available from, as of April 24, 2015: https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=6513e46b-3229-685b-c83b-2209454fae71
/BOXED WARNING/ WARNING: Gastrointestinal Risk: NSAIDs cause an increased risk of serious gastrointestinal adverse events including bleeding, ulceration, and perforation of the stomach or intestines, which can be fatal. These events can occur at any time during use and without warning symptoms. Elderly patients are at greater risk for serious gastrointestinal events.
US Natl Inst Health; DailyMed. Current Medication Information for Meloxicam (meloxicam) tablets (Updated: May 2010). Available from, as of April 24, 2015: https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=6513e46b-3229-685b-c83b-2209454fae71
Contraindications: Known hypersensitivity to meloxicam or any ingredient in the formulation. History of urticaria, angioedema, bronchospasm, severe rhinitis, or shock precipitated by aspirin or other NSAIAs. History of aspirin triad (aspirin sensitivity, asthma, and nasal polyps). Treatment of perioperative pain in the setting of coronary artery bypass graft (CABG) surgery.
American Society of Health System Pharmacists; AHFS Drug Information 2009. Bethesda, MD. (2009), p. 2162
Selective COX-2 inhibitors have been associated with an increased risk of serious adverse cardiovascular thrombotic events in certain situations. Several prototypical NSAIAs also have been associated with an increased risk of cardiovascular events. Findings from a recent systematic review of controlled observational studies and a meta-analysis of published and unpublished data from randomized studies of these agents suggest that use of celecoxib (dosage exceeding 200 mg daily), diclofenac, or indomethacin is associated with an increased risk of cardiovascular events. The possibility exists that meloxicam and ibuprofen also are associated with increased cardiovascular risk.
American Society of Health System Pharmacists; AHFS Drug Information 2009. Bethesda, MD. (2009), p. 2162
For more Drug Warnings (Complete) data for Meloxicam (25 total), please visit the HSDB record page.
Meloxicam is indicated for the symptomatic treatment of arthritis and osteoarthritis. In addition, it is indicated for the pauciarticular and polyarticular course of Juvenile Rheumatoid Arthritis (JRA) in patients aged 2 years old or above. Off-label uses include the treatment of dental or post-surgical pain. In addition to the above, meloxicam has also been studied in the treatment of neuropathic pain. Meloxicam, together with the local anesthetic [bupivacaine], is indicated for the production of postsurgical analgesia in adult patients for up to 72 hours following bunionectomy, open inguinal herniorrhaphy or total knee arthroplasty.
FDA Label
* Cattle:
For use in acute respiratory infection with appropriate antibiotic therapy to reduce clinical signs. For use in diarrhoea in combination with oral re-hydration therapy to reduce clinical signs in calves of over one week of age and young, non-lactating cattle. For adjunctive therapy in the treatment of acute mastitis, in combination with antibiotic therapy. For the relief of post-operative pain following dehorning in calves.
* Pigs:
For the reduction of symptoms of lameness and inflammation in non-infectious locomotor disorders and for adjunctive therapy in the treatment of puerperal septicaemia and toxaemia (mastitis-metritis-agalactia syndrome) with appropriate antibiotic therapy.
* Horses:
For use in the alleviation of inflammation and relief of pain in both acute and chronic musculo-skeletal disorders. For the relief of pain associated with equine colic.
* Cattle:
For use in acute respiratory infection with appropriate antibiotic therapy to reduce clinical signs. For use in diarrhoea in combination with oral rehydration therapy to reduce clinical signs in calves of over one week of age and young, non-lactating cattle. For adjunctive therapy in the treatment of acute mastitis, in combination with antibiotic therapy.
* Pigs:
For use in noninfectious locomotor disorders to reduce the symptoms of lameness and inflammation. For adjunctive therapy in the treatment of puerperal septicaemia and toxaemia (mastitits-metritis-agalactia syndrome) with appropriate antibiotic therapy.
* Horses:
For use in the alleviation of inflammation and relief of pain in both acute and chronic musculoskeletal disorders. For the relief of pain associated with equine colic.
* Dogs: :
Alleviation of inflammation and pain in both acute and chronic musculo-skeletal disorders. Reduction of post-operative pain and inflammation following orthopaedic and soft tissue surgery.
* Cats: :
Reduction of post-operative pain after ovariohysterectomy and minor soft tissue surgery.
* Dogs: : alleviation of inflammation and pain in both acute and chronic musculo-skeletal disorders.
To reduce post-operative pain and inflammation following orthopaedic and soft tissue surgery.
* Cats: : to reduce post-operative pain after ovariohysterectomy and minor soft tissue surgery.
Alleviation of mild to moderate post-operative pain and inflammation following surgical procedures in cats, e. g. orthopaedic and soft tissue surgery.
Alleviation of pain and inflammation in acute and chronic musculo-skeletal disorders in cats.
* Cattle: : for use in acute respiratory infection with appropriate antibiotic therapy to reduce clinical signs.
For use in diarrhoea in combination with oral rehydration therapy to reduce clinical signs in calves of over one week of age and young, non-lactating cattle.
For adjunctive therapy in the treatment of acute mastitis, in combination with antibiotic therapy.
For the relief of post-operative pain following dehorning in calves.
* Pigs: : for use in non-infectious locomotor disorders to reduce the symptoms of lameness and inflammation.
For adjunctive therapy in the treatment of puerperal septicaemia and toxaemia (mastitis-metritis-agalactia syndrome) with appropriate antibiotic therapy.
For the relief of post operative pain associated with minor soft tissue such as castration.
* Horses: : alleviation of inflammation and relief of pain in both acute and chronic musculo-skeletal disorders.
For the relief of pain associated with equine colic.
* Dogs:
Alleviation of inflammation and pain in both acute and chronic musculo-skeletal disorders in dogs. To reduce post-operative pain and inflammation following orthopaedic and soft tissue surgery.
* Cats:
Reduction of post-operative pain after ovariohysterectomy and minor soft tissue surgery.
Alleviation of mild to moderate post-operative pain and inflammation following surgical procedures in cats, e. g. orthopaedic and soft tissue surgery.
Alleviation of pain and inflammation in acute and chronic musculo-skeletal disorders in cats.
* Cattle:
For use in acute respiratory infection with appropriate antibiotic therapy to reduce clinical signs. For use in diarrhoea in combination with oral re-hydration therapy to reduce clinical signs in calves of over one week of age and young, non-lactating cattle. For adjunctive therapy in the treatment of acute mastitis, in combination with antibiotic therapy.
* Pigs:
For use in non-infectious locomotor disorders to reduce the symptoms of lameness and inflammation. For adjunctive therapy in the treatment of puerperal septicaemia and toxaemia (mastitis-metritis-agalactia syndrome) with appropriate antibiotic therapy. For the relief of post operative pain associated with minor soft tissue such as castration.
* Horses:
Alleviation of inflammation and relief of pain in both acute and chronic musculo-skeletal disorders in horses. .
For the relief of pain associated with equine colic.
* Cats:
Alleviation of inflammation and pain in chronic musculoskeletal disorders.
* Dogs:
Alleviation of inflammation and pain in both acute and chronic musculoskeletal disorders.
Alleviation of inflammation and relief of pain in both acute and chronic musculo-skeletal disorders in horses.
* Dogs:
- Alleviation of inflammation and pain in both acute and chronic musculoskeletal disorders.
- Reduction of postoperative pain and inflammation following orthopaedic and soft-tissue surgery.
* Cats:
- Reduction of postoperative pain after ovariohysterectomy and minor soft-tissue surgery.
* Cattle:
- For use in acute respiratory infection with appropriate antibiotic therapy to reduce clinical signs.
- For use in diarrhoea in combination with oral rehydration therapy to reduce clinical signs in calves of over one week of age and young, non-lactating cattle.
- For adjunctive therapy in the treatment of acute mastitis, in combination with antibiotic therapy.
* Pigs:
- For use in noninfectious locomotor disorders to reduce the symptoms of lameness and inflammation.
- For the relief of postoperative pain associated with minor soft-tissue surgery such as castration.
- For adjunctive therapy in the treatment of puerperal septicaemia and toxaemia (mastitis-metritis-agalactia syndrome) with appropriate antibiotic therapy.
* Horses:
- For use in the alleviation of inflammation and relief of pain in both acute and chronic musculoskeletal disorders.
- For the relief of pain associated with equine colic.
* Cattle:
- For use in acute respiratory infection with appropriate antibiotic therapy to reduce clinical signs in cattle.
- For use in diarrhoea in combination with oral rehydration therapy to reduce clinical signs in calves of over one week of age and young, non-lactating cattle.
- For adjunctive therapy in the treatment of acute mastitis, in combination with antibiotic therapy.
* Pigs:
- For use in noninfectious locomotor disorders to reduce the symptoms of lameness and inflammation.
- For adjunctive therapy in the treatment of puerperal septicaemia and toxaemia (mastitis-metritis-agalactia syndrome) with appropriate antibiotic therapy.
* Horses:
- For use in the alleviation of inflammation and relief of pain in both acute and chronic musculoskeletal disorders.
- For the relief of pain associated with equine colic.
Dogs:
- Alleviation of inflammation and pain in both acute and chronic musculo-skeletal disorders in dogs.
Cats:
- Alleviation of mild to moderate post-operative pain and inflammation following surgical procedures in cats, e. g. orthopaedic and soft tissue surgery.
- Alleviation of pain and inflammation in chronic musculo-skeletal disorders in cats.
Guinea pigs:
- Alleviation of mild to moderate post-operative pain associated with soft tissue surgery such as male castration.
* Dogs:
Alleviation of inflammation and pain in both acute and chronic musculoskeletal disorders. To reduce postoperative pain and inflammation following orthopaedic and soft-tissue surgery.
* Cats:
Alleviation of inflammation and pain in chronic musculoskeletal disorders in cats. To reduce postoperative pain after ovariohysterectomy and minor soft-tissue surgery.
* Cattle:
For use in acute respiratory infection with appropriate antibiotic therapy to reduce clinical signs in cattle. For use in diarrhoea in combination with oral rehydration therapy to reduce clinical signs in calves of over one week of age and young, non-lactating cattle. For adjunctive therapy in the treatment of acute mastitis, in combination with antibiotic therapy.
* Pigs:
For use in noninfectious locomotor disorders to reduce the symptoms of lameness and inflammation. For adjunctive therapy in the treatment of puerperal septicaemia and toxaemia (mastitis-metritis-agalactia syndrome) with appropriate antibiotic therapy.
* Horses:
For use in the alleviation of inflammation and relief of pain in both acute and chronic musculoskeletal disorders.
For the relief of pain associated with equine colic.
* Oral suspension: :
Dogs:
Alleviation of inflammation and pain in both acute and chronic musculoskeletal disorders.
* Solution for injection: :
Dogs:
Alleviation of inflammation and pain in both acute and chronic musculoskeletal disorders.
Reduction of postoperative pain and inflammation following orthopaedic and soft-tissue surgery.
Cats:
Reduction of postoperative pain after ovariohysterectomy and minor soft-tissue surgery.
* Dogs:
Alleviation of inflammation and pain in both acute and chronic musculoskeletal disorders.
Reduction of post-operative pain and inflammation following orthopaedic and soft-tissue surgery.
* Cats:
Reduction of post-operative pain after ovariohysterectomy and minor soft-tissue surgery.
* Cattle:
For use in acute respiratory infection with appropriate antibiotic therapy to reduce clinical signs in cattle.
For use in diarrhoea in combination with oral rehydration therapy to reduce clinical signs in calves of over one week of age and young, non-lactating cattle.
For adjunctive therapy in the treatment of acute mastitis, in combination with antibiotic therapy.
* Pigs:
For use in non-infectious locomotor disorders to reduce the symptoms of lameness and inflammation.
For adjunctive therapy in the treatment of puerperal septicaemia and toxaemia (mastitis-metritis-agalactia syndrome) with appropriate antibiotic therapy.
* Horses:
For use in the alleviation of inflammation and relief of pain in both acute and chronic musculoskeletal disorders.
For the relief of pain associated with equine colic.
* Cats: :
- Alleviation of mild to moderate post-operative pain and inflammation following surgical procedures, e. g. orthopaedic and soft tissue surgery.
- Alleviation of pain and inflammation in acute and chronic musculo-skeletal disorders.
- Reduction of post-operative pain after ovariohysterectomy and minor soft tissue surgery.
* Cattle: :
- For use in acute respiratory infection with appropriate antibiotic therapy to reduce clinical signs.
- For use in diarrhoea in combination with oral re-hydration therapy to reduce clinical signs in calves of over one week of age and young, non-lactating cattle.
- For the relief of post-operative pain following dehorning in calves.
- For adjunctive therapy in the treatment of acute mastitis, in combination with antibiotic therapy.
* Dogs: :
- Alleviation of inflammation and pain in both acute and chronic musculo-skeletal disorders.
- Reduction of post-operative pain and inflammation following orthopaedic and soft tissue surgery.
* Horses: :
- For use in the alleviation of inflammation and relief of pain in both acute and chronic musculo-skeletal disorders.
- For the relief of pain associated with equine colic.
- Alleviation of inflammation and relief of pain in both acute and chronic musculo-skeletal disorders.
* Pigs: :
- For use in non-infectious locomotor disorders to reduce the symptoms of lameness and inflammation.
- For the relief of post-operative pain associated with minor soft tissue surgery such as castration.
- For adjunctive therapy in the treatment of puerperal septicaemia and toxaemia (mastitis-metritis-agalactia syndrome) with appropriate antibiotic therapy.
* Guinea pigs: :
- Alleviation of mild to moderate post-operative pain associated with soft tissues surgery such as male castration.
* Novem 5-mg/ml solution for injection for cattle and pigs: :
Cattle
- For use in acute respiratory infection with appropriate antibiotic therapy to reduce clinical signs in cattle.
- For use in diarrhoea in combination with oral rehydration therapy to reduce clinical signs in calves of over one week of age and young, non-lactating cattle.
- For the relief of postoperative pain following dehorning in calves.
Pigs
- For use in noninfectious locomotor disorders to reduce the symptoms of lameness and inflammation.
- For the relief of postoperative pain associated with minor soft-tissue surgery such as castration.
* Novem 20-mg/ml solution for injection for cattle and pigs: :
Cattle
- For use in acute respiratory infection with appropriate antibiotic therapy to reduce clinical signs in cattle.
- For use in diarrhoea in combination with oral rehydration therapy to reduce clinical signs in calves of over one week of age and young, non-lactating cattle.
- For adjunctive therapy in the treatment of acute mastitis, in combination with antibiotic therapy.
- For the relief of postoperative pain following dehorning in calves.
Pigs
- For use in noninfectious locomotor disorders to reduce the symptoms of lameness and inflammation.
- For adjunctive therapy in the treatment of puerperal septicaemia and toxaemia (mastitis-metritis-agalactia syndrome) with appropriate antibiotic therapy.
* Novem 40 mg/ml solution for injection for cattle: :
- For use in acute respiratory infection with appropriate antibiotic therapy to reduce clinical signs in cattle.
- For use in diarrhoea in combination with oral re-hydration therapy to reduce clinical signs in calves of over one week of age and young, non-lactating cattle.
- For adjunctive therapy in the treatment of acute mastitis, in combination with antibiotic therapy.
Alleviation of inflammation and pain in both acute and chronic musculoskeletal disorders in dogs.
* Cattle:
For use in acute respiratory infection with appropriate antibiotic therapy to reduce clinical signs in cattle. For use in diarrhoea in combination with oral rehydration therapy to reduce clinical signs in calves of over one week of age and young, non-lactating cattle. For adjunctive therapy in the treatment of acute mastitis, in combination with antibiotic therapy.
* Pigs:
For use in noninfectious locomotor disorders to reduce the symptoms of lameness and inflammation. For the relief of postoperative pain associated with minor soft-tissue surgery such as castration. For adjunctive therapy in the treatment of puerperal septicaemia and toxaemia (mastitis-metritis-agalactia syndrome) with appropriate antibiotic therapy.
* Horses:
For use in the alleviation of inflammation and relief of pain in both acute and chronic musculoskeletal disorders. For the relief of pain associated with equine colic.
Alleviation of inflammation and pain in both acute and chronic musculoskeletal disorders.
* Oral suspension: :
Dogs:
Alleviation of inflammation and pain in both acute and chronic musculo-skeletal disorders.
* Solution for injection: :
Dogs:
Alleviation of inflammation and pain in both acute and chronic musculo-skeletal disorders.
Reduction of post-operative pain and inflammation following orthopaedic and soft tissue surgery.
Cats:
Reduction of postoperative pain after ovariohysterectomy and minor soft tissue surgery
Meloxicam is an anti-inflammatory, analgesic analgesic with antipyretic effects in fever. Prostaglandins are substances that contribute to inflammation. This drug also exerts preferential actions against COX-2, which may reduce the possible gastrointestinal effects of this drug. In humans, meloxicam has demonstrated the ability to decrease erythrocyte sedimentation rate(ESR) in patients with rheumatoid arthritis, and to decrease ESR, C-reactive protein (CRP), as well as aquaporin-1 expression. As with other NSAIDS, prolonged use of meloxicum can result in renal or cardiovascular impairment or thrombotic cardiovascular events. A note on gastrointestinal effects As meloxicam preferentially inhibits COX-2, it is thought to cause less gastrointestinal irritation compared to other NSAIDS. Despite this, it still carries a risk of gastric inflammation, bleeding and ulceration. In one study, patients on meloxicam suffered from gastrointestinal symptoms at a rate of 13% compared to 19% of those on [diclofenac]. GI events were found to be less severe in the meloxicam-treated patients.
Anti-Inflammatory Agents, Non-Steroidal
Anti-inflammatory agents that are non-steroidal in nature. In addition to anti-inflammatory actions, they have analgesic, antipyretic, and platelet-inhibitory actions. They act by blocking the synthesis of prostaglandins by inhibiting cyclooxygenase, which converts arachidonic acid to cyclic endoperoxides, precursors of prostaglandins. Inhibition of prostaglandin synthesis accounts for their analgesic, antipyretic, and platelet-inhibitory actions; other mechanisms may contribute to their anti-inflammatory effects. (See all compounds classified as Anti-Inflammatory Agents, Non-Steroidal.)
Cyclooxygenase 2 Inhibitors
A subclass of cyclooxygenase inhibitors with specificity for CYCLOOXYGENASE-2. (See all compounds classified as Cyclooxygenase 2 Inhibitors.)
QM01AC06
QM01AC06
QM01AC06
QM01AC06
QM01AC06
QM01AC06
QM01AC06
QM01AC06
QM01AC06
QM01AC06
QM01AC06
QM01AC06
QM01AC06
QM01AC06
QM01AC06
QM01AC06
QM01AC06
QM01AC06
M - Musculo-skeletal system
M01 - Antiinflammatory and antirheumatic products
M01A - Antiinflammatory and antirheumatic products, non-steroids
M01AC - Oxicams
M01AC06 - Meloxicam
Absorption
The absolute bioavailability oral capsules after a dose was 89% in one pharmacokinetic study. Cmax was reached 56 hours after administration of a single dose given after the first meal of the day. The Cmax doubled when the drug was administered in the fasting state. Despite this, meloxicam can be taken without regard to food, unlike many other NSAIDS. Meloxicam formulated for instillation with [bupivacaine] produced varied systemic measures following a single dose of varying strength. In patients undergoing bunionectomy, 1.8 mg of meloxicam produced a Cmax of 26 14 ng/mL, a median Tmax of 18 h, and an AUC of 2079 1631 ng\*h/mL. For a 9 mg dose used in herniorrhaphy, the corresponding values were 225 96 ng/mL, 54 h, and the AUC was not reported. Lastly, a 12 mg dose used in total knee arthroplasty produced values of 275 134 ng/mL, 36 h, and 25,673 17,666 ng\*h/mL.
Route of Elimination
Meloxicam is mainly eliminated through metabolism. Its metabolites are cleared through renal and fecal elimination. Less than <0.25% of a dose is eliminated in the urine as unchanged drug. About 1.6% of the parent drug is excreted in the feces.
Volume of Distribution
The volume of distribution of meloxicam is 10-15L. Because of its high binding to albumin, it is likely to be distributed in highly perfused tissues, such as the liver and kidney. Meloxicam concentrations in synovial fluid, measured after an oral dose, is estimated at 40% to 50% of the concentrations measured in the plasma. This drug is known to cross the placenta in humans.
Clearance
After an oral dose, the total clearance of meloxicam is 0.420.48 L/h. The FDA label indicates a plasma clearance from 7 to 9 mL/min. No dose changes are required in mild to moderate renal or hepatic impairment. The use of meloxicam in patients with severe renal or hepatic impairment has not been studied. FDA prescribing information advises against it.
The absolute bioavailability of meloxicam capsules was 89% following a single oral dose of 30 mg compared with 30 mg iv bolus injection. Following single intravenous doses, dose-proportional pharmacokinetics were shown in the range of 5 mg to 60 mg. After multiple oral doses the pharmacokinetics of meloxicam capsules were dose-proportional over the range of 7.5 mg to 15 mg. Mean Cmax was achieved within four to five hours after a 7.5 mg meloxicam tablet was taken under fasted conditions, indicating a prolonged drug absorption. With multiple dosing, steady state concentrations were reached by Day 5. A second meloxicam concentration peak occurs around 12 to 14 hours post-dose suggesting biliary recycling.
US Natl Inst Health; DailyMed. Current Medication Information for Meloxicam (meloxicam) tablets (September 2007). Available from, as of June 29, 2009: https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?id=5437
Administration of meloxicam capsules following a high fat breakfast (75 g of fat) resulted in mean peak drug levels (ie, Cmax) being increased by approximately 22% while the extent of absorption (AUC) was unchanged. The time to maximum concentration (Tmax) was achieved between 5 and 6 hours.
US Natl Inst Health; DailyMed. Current Medication Information for Meloxicam (meloxicam) tablets (September 2007). Available from, as of June 29, 2009: https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?id=5437
The mean volume of distribution (Vss) of meloxicam is approximately 10 L. Meloxicam is about 99.4% bound to human plasma proteins (primarily albumin) within the therapeutic dose range. The fraction of protein binding is independent of drug concentration, over the clinically relevant concentration range, but decreases to about 99% in patients with renal disease.
US Natl Inst Health; DailyMed. Current Medication Information for Meloxicam (meloxicam) tablets (September 2007). Available from, as of June 29, 2009: https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?id=5437
Meloxicam penetration into human red blood cells, after oral dosing, is less than 10%. Following a radiolabeled dose, over 90% of the radioactivity detected in the plasma was present as unchanged meloxicam.
US Natl Inst Health; DailyMed. Current Medication Information for Meloxicam (meloxicam) tablets (September 2007). Available from, as of June 29, 2009: https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?id=5437
For more Absorption, Distribution and Excretion (Complete) data for Meloxicam (14 total), please visit the HSDB record page.
Meloxicam is almost completely metabolized. CYP2C9 is the main enzyme responsible for the metabolism of meloxicam with minor contributions from CYP3A4. Meloxicam has 4 major metabolites with no activity determined. About 60% of the ingested dose is metabolized to 5'-carboxy meloxicam from hepatic cytochrome enzyme oxidation of an intermediate metabolite, 5-hydroxymethylmeloxicam. Two other metabolites are likely produced via peroxidation.
Meloxicam is almost completely metabolized to four pharmacologically inactive metabolites. The major metabolite, 5'-carboxy meloxicam (60% of dose), from P-450 mediated metabolism was formed by oxidation of an intermediate metabolite 5'-hydroxymethyl meloxicam which is also excreted to a lesser extent (9% of dose). In vitro studies indicate that cytochrome P-450 2C9 plays an important role in this metabolic pathway with a minor contribution of the CYP 3A4 isozyme.
US Natl Inst Health; DailyMed. Current Medication Information for Meloxicam (meloxicam) tablets (September 2007). Available from, as of June 29, 2009: https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?id=5437
Patients' peroxidase activity is probably responsible for /two other/ metabolites which account for 16% and 4% of the administered dose, respectively.
US Natl Inst Health; DailyMed. Current Medication Information for Meloxicam (meloxicam) tablets (September 2007). Available from, as of June 29, 2009: https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?id=5437
Meloxicam is extensively metabolized to inactive metabolites in the liver, principally via the cytochrome P-450 (CYP) 2C9 isoenzyme, with minor contribution by CYP3A4. The drug and its metabolites are excreted in urine and feces, and meloxicam undergoes substantial biliary secretion and enterohepatic recirculation.
American Society of Health System Pharmacists; AHFS Drug Information 2009. Bethesda, MD. (2009), p. 2163
The metabolism of Meloxicam (ME) and the cytochrome(s) P450 (CYPs) involved were analysed by using primary human hepatocytes, human liver microsomes and microsomes from recombinant human B-lymphoblastoid cell lines. While human hepatocytes were capable of converting ME to a 5-hydroxymethyl metabolite (M7) and then to a 5-carboxyderivative (M5), human liver microsomes formed mostly only the 5-hydroxymethylderivative. The kinetics of the formation of M7 by human liver microsomes were biphasic with Km = 13.6 +/- 9.5 and 381 +/- 55.2 uM respectively. The corresponding Vmax were 33.7 +/- 24.2 and 143 +/- 83.9 pmol/min/mg protein respectively. CYP2C9 and, to a much lesser extent, CYP3A4 were found to convert ME to M7. The involvement of 2C9 was demonstrated by inhibition of tolbutamide hydroxylase activity in the presence of ME, inhibition of ME metabolism by sulphaphenazole, correlation between ME metabolism and tolbutamide hydroxylase activity and active metabolism of ME by recombinant 2C9. The involvement of 3A4 was shown by inhibition of ME metabolism by ketoconazole, correlation between ME metabolism and nifedipine oxidase activity and metabolism of ME by recombinant 3A4. Kinetics of the formation of M7 by the individual enzymes resulted in a Km = 9.6 uM and Vmax = 8.4 pmol/min/mg protein for 2C9 and a Km = 475 uM and Vmax = 23 pmol/min/mg protein for 3A4.
PMID:9493314 Chesne C et al; Xenobiotica 28 (1): 1-13 (1998).
For more Metabolism/Metabolites (Complete) data for Meloxicam (6 total), please visit the HSDB record page.
Meloxicam has known human metabolites that include 5-Hydroxymethyl meloxicam.
S73 | METXBIODB | Metabolite Reaction Database from BioTransformer | DOI:10.5281/zenodo.4056560
The half-life of meloxicam is approximately 20 hours, which is considerably longer than most other NSAIDS. It can therefore be dosed without the need for slow-release formulations. Meloxicam applied together with [bupivacaine] for postsurgical analgesia had a median half-life of 33-42 hours, depending on dose and application site.
The mean elimination half-life (t1/2) ranges from 15 hours to 20 hours. The elimination half-life is constant across dose levels indicating linear metabolism within the therapeutic dose range.
US Natl Inst Health; DailyMed. Current Medication Information for Meloxicam (meloxicam) tablets (September 2007). Available from, as of June 29, 2009: https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?id=5437
... The twenty volunteers can be classified into extensive metabolizers and poor metabolizers according to pharmacokinetic parameters. The main parameters in the two groups obtained were as follows: T 1/2 were 21 +/- 4 and 38 +/- 9 hr, respectively. ...
PMID:12579866 Xu HY et al; Yao Xue Xue Bao 36 (1): 71-3 (2001).
Meloxicam inhibits prostaglandin synthetase (cylooxygenase 1 and 2) enzymes leading to a decreased synthesis of prostaglandins, which normally mediate painful inflammatory symptoms. As prostaglandins sensitize neuronal pain receptors, inhibition of their synthesis leads to analgesic and inflammatory effects. Meloxicam preferentially inhibits COX-2, but also exerts some activity against COX-1, causing gastrointestinal irritation.
Meloxicam, an oxicam derivative that is structurally related to piroxicam, is a nonsteroidal anti-inflammatory agent (NSAIA) exhibiting analgesic, antipyretic, and anti-inflammatory actions. In vitro and in vivo studies indicate that meloxicam inhibits the cyclooxygenase-2 (COX-2) isoform of prostaglandin endoperoxide synthase (prostaglandin G/H synthase [PGHS]) to a greater extent than the COX-1 isoform. However, meloxicam's COX-2 selectivity is dose dependent and is diminished at higher dosages. Therefore meloxicam sometimes has been referred to as a "preferential" rather than "selective" COX-2 inhibitor.
American Society of Health System Pharmacists; AHFS Drug Information 2009. Bethesda, MD. (2009), p. 2162
To investigate the effect of meloxicam on human polymorphonuclear leukocyte (PMN) adhesion to human synovial cell (HSC), and to explore its mechanism. MTT colorimetry was used to determine the adhesion effect of PMN to HSC. Cell-ELISA and RT-PCR methods were used to determine the expression of ICAM-1 and VCAM-1. Nuclear transcription factor-kappa B (NF-kappa B) was measured by electrophoretic mobility shift assay (EMSA) method. Meloxicam was found to effectively inhibit TNF-alpha (50 u.mL-1 for 12 hr) and IL-1 beta (50 u.mL-1 for 12 hr)-induced adhesion of PMN to HSC (IC50 3.38 X 10(-7) mol.L-1 and 3.56 X 10(-6) mol.L-1, respectively) in a concentration-dependent manner. ICAM-1 protein and mRNA expression induced by TNF-alpha (50 u.mL-1) were inhibited by meloxicam at 1 X 10(-6)-1 X 10(-5) mol.L-1. The activation of NF-kappa B was also inhibited by meloxicam at 1 X 10(-6)-1 X 10(-5) mol.L-1. These results suggest that meloxicam inhibit TNF-alpha stimulated PMN-HSC adhesion and expression of ICAM-1 by suppressing the activity of NF-kappa B.
PMID:12579952 Li LC et al; Yao Xue Xue Bao 37 (2): 103-7 (2002).
/Investigators/ compared the effects of therapeutically equivalent doses of meloxicam and indomethacin, a preferential inhibitor of the constitutive cyclooxygenase (COX-1), on platelet aggregation and platelet thromboxane formation, which are exclusively COX-1 dependent, physiological renal, and total body prostaglandin E2 (PGE2) production. In a randomized cross-over design, 14 healthy female volunteers received meloxicam 7.5 mg per day for 6 days or indomethacin 25 mg three times per day for 3 days; the wash-out period was 5 days, and drug intake was adapted to the menstrual cycle. On the day before treatment and on the last day of each treatment period the following parameters were evaluated: maximum platelet aggregation and thromboxane B2 (TXB2) formation in response to 1.0 mmol/L arachidonic acid; 24-hour urinary excretion of PGE2 and 7 alpha-hydroxy-5, 11-diketo-tetranor-prosta-1, 16-dionic acid (PGE-M), the index metabolites of renal and total body PGE2 synthesis, respectively, were assessed by gas chromatography/tandem mass spectrometry. Maximum platelet aggregation and TXB2 formation were almost completely inhibited by indomethacin (-87% and -99%, respectively; p < 0.001, each) as compared to control (100%), but remained unaffected by meloxicam (-1% and +4%, respectively). Meloxicam showed no significant effects on urinary PGE2 excretion (-13%) and only slight effects on PGE-M excretion (-22%; p < 0.05), whereas indomethacin reduced urinary PGE2 excretion (-43%; p < 0.05) as well as PGE-M excretion (-36%; p < 0.001). /This/ data shows, that meloxicam 7.5 mg per day is COX-1 sparing in humans in vivo.
PMID:9084574 Stichtenoth DO et al; J Investig Med 45 (2): 44-9 (1997).
BUILDING BLOCK

