




1. 120279-26-7
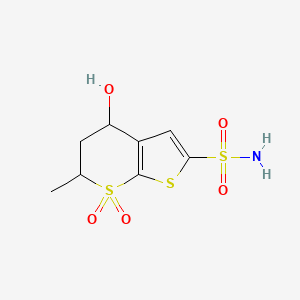
2. 5,6-dihydro-4h-4-hydroxy-6-methyl Thieno [2,3,b] Thiopyran-2-sulfonamide-7,7-dioxide
3. 4-hydroxy-6-methyl-7,7-dioxo-5,6-dihydro-4h-thieno[2,3-b]thiopyran-2-sulfonamide
4. 4h-thieno[2,3-b]thiopyran-2-sulfonamide,5,6-dihydro-4-hydroxy-6-methyl-, 7,7-dioxide
5. 5,6-dihydro-4-hydroxy-6-methyl-4h-thieno(2,3-b)thiopyran-2-sulfonamide 7,7-dioxide
6. Schembl2348864
7. Dtxsid80559768
8. Akos015898688
9. Ft-0659484
10. A804481
11. 4-hydroxy-6-methyl-7,7-dioxo-4,5,6,7-tetrahydro-7lambda~6~-thieno[2,3-b]thiopyran-2-sulfonamide
| Molecular Weight | 297.4 g/mol |
|---|---|
| Molecular Formula | C8H11NO5S3 |
| XLogP3 | -0.2 |
| Hydrogen Bond Donor Count | 2 |
| Hydrogen Bond Acceptor Count | 7 |
| Rotatable Bond Count | 1 |
| Exact Mass | g/mol |
| Monoisotopic Mass | g/mol |
| Topological Polar Surface Area | 160 |
| Heavy Atom Count | 17 |
| Formal Charge | 0 |
| Complexity | 502 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 0 |
| Undefined Atom Stereocenter Count | 2 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 1 |


