

1. Azd-9291
2. Azd-9291 Mesylate
3. Azd9291
4. Azd9291 Mesylate
5. Mereletinib
6. Mereletinib Mesilate
7. Mereletinib Mesylate
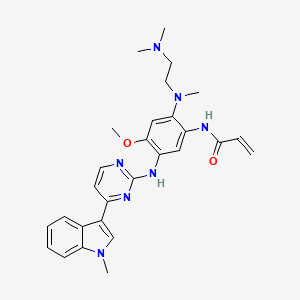
8. N-(2-((2-(dimethylamino)ethyl)methylamino)-4-methoxy-5-((4-(1-methyl-1h-indol-3-yl)-2-pyrimidinyl)amino)phenyl)-2-propenamide
9. N-(2-((2-(dimethylamino)ethyl)methylamino)-4-methoxy-5-((4-(1-methyl-1h-indol-3-yl)-2-pyrimidinyl)amino)phenyl)-2-propenamide Methanesulfonate (1:1)
10. Osimertinib Mesilate
11. Osimertinib Mesylate
12. Tagrisso
1. 1421373-65-0
2. Azd-9291
3. Mereletinib
4. Azd9291
5. Azd 9291
6. Osimertinib [usan]
7. Unii-3c06jj0z2o
8. N-[2-[2-(dimethylamino)ethyl-methylamino]-4-methoxy-5-[[4-(1-methylindol-3-yl)pyrimidin-2-yl]amino]phenyl]prop-2-enamide
9. 3c06jj0z2o
10. Azd-9291 Free Base
11. N-(2-{[2-(dimethylamino)ethyl](methyl)amino}-4-methoxy-5-{[4-(1-methyl-1h-indol-3-yl)pyrimidin-2-yl]amino}phenyl)prop-2-enamide
12. N-(2-((2-(dimethylamino)ethyl)(methyl)amino)-4-methoxy-5-((4-(1-methyl-1h-indol-3-yl)pyrimidin-2-yl)amino)phenyl)acrylamide
13. 2-propenamide, N-(2-((2-(dimethylamino)ethyl)methylamino)-4-methoxy-5-((4-(1-methyl-1h-indol-3-yl)-2-pyrimidinyl)amino)phenyl)-
14. N-(2-{[2-(dimethylamino)ethyl](methyl)amino}-4-methoxy-5-{[4-(1-methylindol-3-yl)pyrimidin-2-yl]amino}phenyl)prop-2-enamide
15. N-[2-[[2-(dimethylamino)ethyl]methylamino]-4-methoxy-5-[[4-(1-methyl-1h-indol-3-yl)-2-pyrimidinyl]amino]phenyl]-2-propenamide
16. Mereletinib [inn]
17. Osimertinibum
18. N-(2-{[2-(dimethylamino)ethyl](methyl)amino}-4-methoxy-5-{[4-(1-methyl-1h-indol-3-yl)pyrimidin-2-yl]amino}phenyl)acrylamide
19. Osimertinib [mi]
20. Osimertinib [inn]
21. Osimertinib (azd9291)
22. Osimertinib; Azd 9291
23. Osimertinib; Azd-9291
24. Mereletinib (obsolete Inn)
25. Osimertinib [who-dd]
26. Gtpl7719
27. Chembl3353410
28. Schembl14660911
29. Chebi:90943
30. Amy9161
31. Ex-a314
32. Mereletinibazd-9291,osimertinib
33. Dtxsid501025961
34. Hms3653e10
35. Hms3672m05
36. Bcp08626
37. Bdbm50029668
38. Mfcd27988062
39. Nsc779217
40. Nsc781254
41. Nsc800812
42. S7297
43. Zinc98023177
44. Akos025290756
45. Ccg-264683
46. Cs-2018
47. Db09330
48. Nsc-779217
49. Nsc-781254
50. Nsc-800812
51. Sb22952
52. Ncgc00378622-03
53. Ncgc00378622-04
54. Ncgc00378622-10
55. Ac-29019
56. As-16943
57. Hy-15772
58. 4714b
59. Sw219863-1
60. A854509
61. Q21506464
62. N(2{[2(dimethylamino)ethyl](methyl)amino}4methoxy5{[4(1methyl1hindol3yl)pyrimidin2yl]amino}phenyl)prop2enamide;osimertinib
63. N-(2-((2-(dimethylamino)ethyl)(methyl)amino)-4-methoxy-5-(4-(1-methyl-1h-indol-3-yl)pyrimidin-2-ylamino)phenyl)acrylamide
64. N-(2-{2-dimethylaminoethyl-methylamino}-4-methoxy-5-{[4-(1-methylindol-3-yl)pyrimidin-2-yl]amino}phenyl)prop-2-enamide
| Molecular Weight | 499.6 g/mol |
|---|---|
| Molecular Formula | C28H33N7O2 |
| XLogP3 | 3.7 |
| Hydrogen Bond Donor Count | 2 |
| Hydrogen Bond Acceptor Count | 7 |
| Rotatable Bond Count | 10 |
| Exact Mass | 499.26957332 g/mol |
| Monoisotopic Mass | 499.26957332 g/mol |
| Topological Polar Surface Area | 87.6 Ų |
| Heavy Atom Count | 37 |
| Formal Charge | 0 |
| Complexity | 752 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 0 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 1 |
| 1 of 1 | |
|---|---|
| Drug Name | TAGRISSO |
| Active Ingredient | OSIMERTINIB MESYLATE |
| Company | ASTRAZENECA PHARMS (Application Number: N208065. Patents: 8946235, 9732058) |
Osimertinib is indicated for the treatment of patients with metastatic epidermal growth factor receptor (EGFR) T790M mutation-positive non-small cell lung cancer (NSCLC), as detected by an FDA- approved test, who have progressed on or after EGFR-TKI therapy.
FDA Label
TAGRISSO as monotherapy is indicated for:
-the adjuvant treatment after complete tumour resection in adult patients with stage IB-IIIA non-small cell lung cancer (NSCLC) whose tumours have epidermal growth factor receptor (EGFR) exon 19 deletions or exon 21 (L858R) substitution mutations
- the first-line treatment of adult patients NSCLC with activating EGFR mutations.
- the treatment of adult patients with locally advanced or metastatic EGFR T790M mutation-positive NSCLC.
TAGRISSO as monotherapy is indicated for:
- the adjuvant treatment after complete tumour resection in adult patients with stage IB-IIIA non-small cell lung cancer (NSCLC) whose tumours have epidermal growth factor receptor (EGFR) exon 19 deletions or exon 21 (L858R) substitution mutations.
- the first-line treatment of adult patients with locally advanced or metastatic NSCLC with activating EGFR mutations.
- the treatment of adult patients with locally advanced or metastatic EGFR T790M mutation-positive NSCLC.
A pharmacokinetic/pharmacodynamic analysis suggested a concentration-dependent QTc interval prolongation of 14 msec (upper bound of two-sided 90% CI: 16 msec) at a dose of osimertinib 80 mg.
Antineoplastic Agents
Substances that inhibit or prevent the proliferation of NEOPLASMS. (See all compounds classified as Antineoplastic Agents.)
Protein Kinase Inhibitors
Agents that inhibit PROTEIN KINASES. (See all compounds classified as Protein Kinase Inhibitors.)
L01XE
L - Antineoplastic and immunomodulating agents
L01 - Antineoplastic agents
L01E - Protein kinase inhibitors
L01EB - Epidermal growth factor receptor (egfr) tyrosine kinase inhibitors
L01EB04 - Osimertinib
Absorption
The median time to Cmax was found to be 6 hours.
Route of Elimination
Osimertinib is primarily eliminated through excretion in the feces (68%), to a lesser extent through urine (14%), while only 2% is excreted unchanged.
Volume of Distribution
The mean volume of distribution at steady state is 986 L.
Clearance
Oral clearance is 14.2 L/hr.
Osimertinib is metabolized to at least two pharmacologically active metabolites, AZ7550 and AZ5104, that circulate at approximately 10% of the concentration of the parent compound. Biochemical assays have shown that AZ7550 has similar potency and efficacy to osimertinib, while AZ5104 is more potent against mutant and wild-type EGFR. The main metabolic pathways are oxidation (predominantly by CYP3A) and dealkylation.
The population estimated mean half-life is 48 hours.
Osimertinib is an epidermal growth factor receptor (EGFR) tyrosine kinase inhibitor (TKI) that binds to certain mutant forms of EGFR (T790M, L858R, and exon 19 deletion) that predominate in non-small cell lung cancer (NSCLC) tumours following treatment with first-line EGFR-TKIs. As a third-generation tyrosine kinase inhibitor, osimertinib is specific for the gate-keeper T790M mutation which increases ATP binding activity to EGFR and results in poor prognosis for late-stage disease. Furthermore, osimertinib has been shown to spare wild-type EGFR during therapy, thereby reducing non-specific binding and limiting toxicity.
BUILDING BLOCK

MARKET PLACE



LOOKING FOR A SUPPLIER?