
1. 143174-36-1
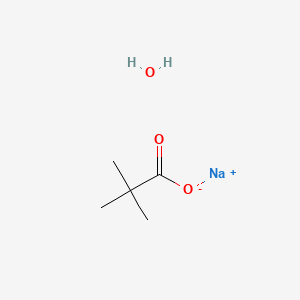
2. Sodium Pivalate Hydrate
3. Sodium;2,2-dimethylpropanoate;hydrate
4. Sodium Trimethylacetate Xhydrate
5. Mfcd00150782
6. Propanoic Acid, 2,2-dimethyl-, Sodium Salt, Hydrate
7. Schembl3751460
8. Dtxsid20635414
9. Sodium Trimethylacetate Hydrate, 99%
10. Akos015903886
11. As-81744
12. Sy011635
13. Db-042670
14. Ft-0643355
15. H10729
16. Sodium 2,2-dimethylpropanoate--water (1/1/1)
| Molecular Weight | 142.13 g/mol |
|---|---|
| Molecular Formula | C5H11NaO3 |
| Hydrogen Bond Donor Count | 1 |
| Hydrogen Bond Acceptor Count | 3 |
| Rotatable Bond Count | 1 |
| Exact Mass | 142.06058849 g/mol |
| Monoisotopic Mass | 142.06058849 g/mol |
| Topological Polar Surface Area | 41.1 Ų |
| Heavy Atom Count | 9 |
| Formal Charge | 0 |
| Complexity | 82.9 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 0 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 3 |
BUILDING BLOCK

