

1. 2-methylimidazole Hydrochloride
2. 2-methylimidazole, Silver (1+) Salt
3. Zn(2-methylimidazole)2
1. 693-98-1
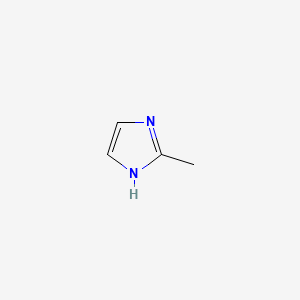
2. 2-methyl-1h-imidazole
3. 1h-imidazole, 2-methyl-
4. Imidazole, 2-methyl-
5. 2-methyl Imidazole
6. 2-methylglyoxaline
7. Mfcd00005190
8. Chembl293391
9. Dtxsid4022107
10. T0049z45lz
11. Nsc 21394-d5
12. Nsc-21394
13. 2mz
14. Ncgc00091456-01
15. Dtxcid202107
16. Methylimidazole
17. 2-methyl-1h-imidazole; 1h-2-methylimidazole; 2-methylimidazole; 2mi; 2mz; 2mz-h
18. Cas-693-98-1
19. Ccris 2459
20. Einecs 211-765-7
21. Nsc 21394
22. Methylimidazol
23. Methyl Imidazol
24. Unii-t0049z45lz
25. 2methylimidazole
26. Methyl-imidazole
27. Ai3-50033
28. 2-methylimidazol
29. 2-metylimidazole
30. 2-methylimdazole
31. Hsdb 7756
32. 2-methyl-imidazol
33. 2-methyl-imidazole
34. 2-methyl Glyoxaline
35. 2-methyl-1h-imidazol
36. Curezol-2mz-p
37. Imicure-ami 2
38. Epicure-mi 2
39. Epikure-mi 2
40. 2-methylimidazole, 99%
41. Ec 211-765-7
42. Methylimidazole, 2-
43. Wln: T5m Cnj B1
44. Mls001065618
45. 2-methylimidazole [hsdb]
46. 2-methylimidazole [iarc]
47. Hms3039f06
48. Nsc21394
49. Str02220
50. Tox21_111136
51. Tox21_201316
52. Tox21_302808
53. Bbl013047
54. Bdbm50295560
55. Stk301707
56. Akos000118836
57. Cs-w019983
58. Pb43299
59. Ncgc00091456-02
60. Ncgc00256531-01
61. Ncgc00258868-01
62. Pd063450
63. Smr000568464
64. 2-methylimidazole (ondansetron Impurity F)
65. Am20100647
66. Ft-0613056
67. Ft-0671849
68. M0345
69. Ns00010845
70. En300-21278
71. C19261
72. D77862
73. A833348
74. A836474
75. W-104630
76. Q21099566
77. F2190-0640
78. Z104494990
79. Inchi=1/c4h6n2/c1-4-5-2-3-6-4/h2-3h,1h3,(h,5,6
80. Ondansetron Hydrochloride Dihydrate Impurity F [ep Impurity]
81. 2-methylimidazole, >=95.0% (hplc), Pharmaceutical Impurity Standard
82. Ondansetron Impurity F, European Pharmacopoeia (ep) Reference Standard
83. Ondansetron Hydrochloride Impurity, 2-methylimidazole- [usp Impurity]
84. 2-methylimidazole (ondansetron Impurity F), Pharmaceutical Secondary Standard; Certified Reference Material
| Molecular Weight | 82.10 g/mol |
|---|---|
| Molecular Formula | C4H6N2 |
| XLogP3 | 0.2 |
| Hydrogen Bond Donor Count | 1 |
| Hydrogen Bond Acceptor Count | 1 |
| Rotatable Bond Count | 0 |
| Exact Mass | g/mol |
| Monoisotopic Mass | g/mol |
| Topological Polar Surface Area | 28.7 |
| Heavy Atom Count | 6 |
| Formal Charge | 0 |
| Complexity | 44.8 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 0 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 1 |
... The disposition of [2-(14)C]-2-methylimidazole (2-MI) has been investigated following po administration of either 5, 50, or 150 mg/kg to male F344 rats. Excretion data indicated that absorption of 2-MI was both rapid and proportional to dose in the range studied. Approximately 90% of the total dose was eliminated in urine within 24 hr. Most of the remaining 14C was excreted in feces and as expired 14CO2. Excretion data were similar following iv administration of 5 mg/kg. Little or no enterohepatic circulation of compound occurred, since biliary excretion of 2-MI-derived 14C was negligible. Approximately 70% of the 14C excreted in urine, following all dosing, consisted of parent compound. High-performance liquid chromatography (HPLC) chromatograms for all treatment groups were similar, indicating that metabolism of 2-MI in rats was not affected by dose or route of administration.
PMID:9652548 Sanders JM et al; J Toxicol Environ Health A 54 (2): 121-32 (1998).
The toxicokinetics of 2-methylimidazole (2-MI) were studied in male and female Fischer 344 rats after a single iv dose of 10 mg/kg or gavage dose of 25, 50, or 100 mg/kg. The 2-MI was formulated in 0.05 M phosphate-buffered saline (pH 7.4). The iv profiles could be best described by a two-compartment model with first-order elimination. The terminal elimination half-life, volume of distribution at steady state, and clearance values were 0.78 and 0.85/hr, 1.5 and 1.9 L, and 4.97 and 12.0 L/hr/kg for males and females, respectively. After a gavage dose, the plasma concentration time profiles could be best described by a one-compartment model, no lag phase, and first-order absorption and elimination. The peak 2-MI plasma concentrations increased proportionately with dose and were reached within 35 to 50 min (T(max)) for all groups. The estimated half-life value for 2-MI was about 1 hr for the iv group and the male 25-, 50-, or 100-mg/kg groups and female 25-mg/kg groups. Clearance increased for the male 100- and female 50- and 100- mg/kg groups. For a given dose group, clearance was also two to three times greater for female rats when compared to male rats. Absolute bioavailability for 2-MI was estimated to approach 97%. The results of this study indicated that 2-MI was (1) rapidly and completely absorbed, (2) quickly eliminated, (3) cleared differently for females than for males, (4) affected somewhat by dose for females, and (5) unlikely to undergo tissue accumulation following repeated exposure.
PMID:12079612 Johnson JD et al; J Toxicol Environ Health A 65 (12): 869-79 (2002).
The toxicokinetics of 2-methylimidazole (2-MI) were studied in male and female Fischer 344 rats after a single iv dose of 10 mg/kg or gavage dose of 25, 50, or 100 mg/kg. The 2-MI was formulated in 0.05 M phosphate-buffered saline (pH 7.4) ... The estimated half-life value for 2-MI was about 1 hr for the iv group and the male 25-, 50-, or 100-mg/kg groups and female 25-mg/kg groups ...
PMID:12079612 Johnson JD et al; J Toxicol Environ Health A 65 (12): 869-79 (2002).
BUILDING BLOCK



LOOKING FOR A SUPPLIER?