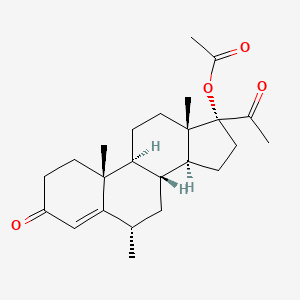

1. (6 Alpha)-17-(acetoxy)-6-methylpregn-4-ene-3,20-dione
2. 6 Alpha Methyl 17alpha Hydroxyprogesterone Acetate
3. 6-alpha-methyl-17alpha-hydroxyprogesterone Acetate
4. Curretab
5. Cycrin
6. Depo Medroxyprogesterone Acetate
7. Depo Provera
8. Depo-medroxyprogesterone Acetate
9. Depo-provera
10. Depoprovera
11. Farlutal
12. Gestapuran
13. Medroxyprogesterone 17 Acetate
14. Medroxyprogesterone 17-acetate
15. Medroxyprogesterone 17-acetate, (6 Alpha,17 Alpha)-isomer
16. Medroxyprogesterone 17-acetate, (6 Beta)-isomer
17. Perlutex
18. Pregn-4-ene-3,20-dione, 17-(acetyloxy)-6-methyl-, (6alpha)-
19. Provera
20. Veramix
1. Medroxyprogesterone 17-acetate
2. 71-58-9
3. Provera
4. Metigestrona
5. Depo-provera
6. Farlutin
7. Gestapuran
8. Perlutex
9. Veramix
10. Methylacetoxyprogesterone
11. Amen
12. Medroxyacetate Progesterone
13. Clinovir
14. Depcorlutin
15. Deporone
16. Lutopolar
17. Nadigest
18. Prodasone
19. Progestalfa
20. Progevera
21. Proverone
22. Repromix
23. Sirprogen
24. Supprestral
25. Nidaxin
26. Oragest
27. Depo-promone
28. Promone-e
29. Nsc-26386
30. 6-alpha-methyl-17-alpha-acetoxyprogesterone
31. Cycrin
32. Medroxyprogesteroneacetate
33. Depo-subq Provera 104
34. Depot-medroxyprogesterone Acetate
35. 6-alpha-methyl-17-alpha-hydroxyprogesterone Acetate
36. 17alpha-hydroxy-6alpha-methylprogesterone Acetate
37. 6alpha-methyl-17alpha-hydroxyprogesterone Acetate
38. 6alpha-methyl-4-pregnene-3,20-dion-17alpha-ol Acetate
39. Depot Medroxyprogesterone Acetate
40. Dp150
41. Pregn-4-ene-3,20-dione, 17-(acetyloxy)-6-methyl-, (6alpha)-
42. 17-acetoxy-6alpha-methylprogesterone
43. Aragest
44. Ralovera
45. 6alpha-methyl-17-acetoxy Progesterone
46. Hysron
47. Provera Dosepak
48. Depo-clinovir
49. Depo-ralovera
50. (6alpha)-6-methyl-3,20-dioxopregn-4-en-17-yl Acetate
51. Nsc-21171
52. U 8839
53. C2qi4ioi2g
54. Medroxyprogesterone (acetate)
55. Mls000069442
56. (6alpha)-17-(acetyloxy)-6-methylpreg-4-ene-3,20-dione
57. Chebi:6716
58. Onco-provera
59. Map
60. Medrosterona
61. Clinofem
62. Cykrina
63. Depocon
64. Indivina
65. Mepastat
66. Meprate
67. Repromap
68. Smr000059125
69. Sumiferm
70. Suprestral
71. Tv-46046
72. Veraplex
73. Dugen
74. Pregn-4-ene-3,20-dione, 17-(acetyloxy)-6-methyl-, (6a)-
75. Depo-progestin
76. Depo-prodasone
77. Depo-progevera
78. Perlutex Leo
79. Dsstox_cid_5527
80. Depo-map
81. Aragest 5
82. Med-pro
83. Mpa-beta
84. Dsstox_rid_77819
85. Mpa Hexal
86. Mpa-noury
87. Dsstox_gsid_25527
88. [(6s,8r,9s,10r,13s,14s,17r)-17-acetyl-6,10,13-trimethyl-3-oxo-2,6,7,8,9,11,12,14,15,16-decahydro-1h-cyclopenta[a]phenanthren-17-yl] Acetate
89. Map (steroid)
90. 17alpha-acetoxy-6alpha-methylprogesterone
91. 6alpha-methyl-17alpha-acetoxyprogesterone
92. Depomedroxyprogesterone Acetate
93. Mpa Gym
94. Depo-medroxyprogesterone Acetate
95. Depo-subq Provera
96. Cas-71-58-9
97. Ccris 371
98. 17.alpha.-acetoxy-6.alpha.-methylprogesterone
99. Medroxyprogesterone Acetate [jan]
100. Einecs 200-757-9
101. Unii-c2qi4ioi2g
102. 6alpha-methyl-17-acetoxyprogesterone
103. Nsc 21171
104. Depo-provera Contraceptive
105. 17-acetoxy-6-alpha-methylprogesterone
106. (6.alpha.-pregn-4-ene-3, 17-(acetyloxy)-6-methyl-
107. 17.alpha.-hydroxy-6-.alpha.-methylprogesterone Acetate
108. Pregn-4-ene-3, 17-hydroxy-6.alpha.-methyl-, Acetate
109. 6-alpha-methyl-17-acetoxy Progesterone
110. Brn 2066112
111. Pregn-4-ene-3, 17-(acetyloxy)-6-methyl-, (6.alpha.)-
112. Medroxyprogesterone-17-acetate
113. Ai3-60127
114. Pregn-4-ene-3,20-dione, 17-(acetyloxy)-6-methyl-, (6.alpha.)-
115. 17-acetoxy-6.alpha.-methylprogesterone
116. 6.alpha.-methyl-17-acetoxyprogesterone
117. Ncgc00094713-01
118. 17alpha-hydroxy-6-alpha-methylprogesterone Acetate
119. Progesterone, 17-hydroxy-6alpha-methyl-, Acetate
120. 17-alpha-hydroxy-6-alpha-methylprogesterone Acetate
121. Provera (tn)
122. Depo-provera (tn)
123. 6alpha-methyl-17alpha-acetoxypregn-4-ene-3,20-dione
124. Medroxyprogesterone Acetate [usp:jan]
125. 17-alpha-acetoxy-6-alpha-methylpregn-4-ene-3,20-dione
126. 17alpha-acetoxy-6-alpha-methylpregn-4-ene-3,20-dione
127. 6-alpha-methyl-17-alpha-acetoxypregn-4-ene-3,20-dione
128. Progesterone, 17-alpha-hydroxy-6-alpha-methyl-, Acetate
129. Medroxyprogesterone Acetate [progestins]
130. 6-alpha-methyl-4-pregnene-3,20-dion-17-alpha-ol Acetate
131. 6.alpha.-methyl-17.alpha.-acetoxyprogesterone
132. (6-alpha)-17-(acetyloxy)-6-methylpreg-4-ene-3,20-dione
133. 17alpha-hydroxy-6alpha-methylpregn-4-ene-3,20-dione Acetate
134. 6alpha-pregn-4-ene-3,20-dione, 17-(acetyloxy)-6-methyl-
135. Cpd000653524
136. 17-alpha-hydroxy-6-alpha-methylpregn-4-ene-3,20-dione Acetate
137. Medroxyprogesteroni Acetas
138. Opera_id_1110
139. Medroxyprogesterone-acetate
140. 17.alpha.-hydroxy-6.alpha.-methylprogesterone Acetate
141. 6.alpha.-methyl-17.alpha.-hydroxyprogesterone Acetate
142. Medroxiprogesterone Acetate
143. Chembl717
144. Schembl4276
145. 6.alpha.-methyl-17.alpha.-acetoxypregn-4-ene-3,20-dione
146. 17.alpha.-acetoxy-6-.alpha.-methylpregn-4-ene-3,20-dione
147. 4-08-00-02212 (beilstein Handbook Reference)
148. 6.alpha.-methyl-4-pregnene-3,20-dion-17.alpha.-ol Acetate
149. Mls001148217
150. Mls002207115
151. 17.alpha.-hydroxy-6.alpha.-methylpregn-4-ene-3,20-dione Acetate
152. Depo-subq Provera 104 (tn)
153. Dtxsid0025527
154. Hms2233o07
155. Hms2235e05
156. Hms3259o14
157. Hms3884b10
158. Pregn-4-ene-3,20-dione, 17-hydroxy-6alpha-methyl-, Acetate
159. (6-alpha)-pregn-4-ene-3,20-dione, 17-(acetyloxy)-6-methyl-
160. (6s,8r,9s,10r,13s,14s,17r)-17-acetyl-6,10,13-trimethyl-3-oxo-2,3,6,7,8,9,10,11,12,13,14,15,16,17-tetradecahydro-1h-cyclopenta[a]phenanthren-17-yl Acetate
161. Cbp-1011
162. Hy-b0469
163. Nsc21171
164. Nsc26386
165. Zinc5029557
166. Tox21_111319
167. Tox21_200141
168. Bdbm50067678
169. S2567
170. Akos015894870
171. Tox21_111319_1
172. Ac-2174
173. Ccg-264965
174. Db00603
175. Nc00574
176. Medroxyprogesterone Acetate (jp17/usp)
177. Mrf-0000023
178. Ncgc00022037-03
179. Ncgc00022037-04
180. Ncgc00022037-05
181. Ncgc00022037-07
182. Ncgc00257695-01
183. Ncgc00263480-01
184. As-13981
185. Medroxyprogesterone Acetate [iarc]
186. Smr000653524
187. Medroxyprogesterone Acetate [mart.]
188. Medroxyprogesterone Acetate [vandf]
189. Medroxyprogesterone 17-acetate [mi]
190. Medroxyprogesterone Acetate [usp-rs]
191. Medroxyprogesterone Acetate [who-dd]
192. Medroxyprogesterone Acetate [who-ip]
193. C08150
194. C76275
195. D00951
196. Ab00384270-14
197. Ab00384270_15
198. Medroxyprogesterone 17-acetate, >=97% (hplc)
199. Medroxyprogesterone Acetate [orange Book]
200. Medroxyprogesteroni Acetas [who-ip Latin]
201. 010m483
202. Medroxyprogesterone Acetate [ep Monograph]
203. Medroxyprogesterone Acetate [usp Monograph]
204. Megestrol Acetate Impurity A [ep Impurity]
205. Lunelle Component Medroxyprogesterone Acetate
206. Prempro Component Medroxyprogesterone Acetate
207. Q2823834
208. (6?)-6-methyl-3,20-dioxopregn-4-en-17-yl Acetate
209. 6.alpha.-pregn-4-ene-3, 17-(acetyloxy)-6-methyl-
210. Medroxyprogesterone Acetate Component Of Lunelle
211. Medroxyprogesterone Acetate Component Of Prempro
212. Premphase Component Medroxyprogesterone Acetate
213. Medroxyprogesterone Acetate Component Of Premphase
214. Medroxyprogesterone 17-acetate 100 Microg/ml In Methanol/water
215. Medroxyprogesterone-17-acetate 100 Microg/ml In Acetonitrile
216. 17-hydroxy-6.alpha.-methylpregn-4-ene-3,20-dione Acetate
217. Medroxyprogesterone 17-acetate, Vetranal(tm), Analytical Standard
218. Medroxyprogesterone Acetate, European Pharmacopoeia (ep) Reference Standard
219. Medroxyprogesterone Acetate, Pharmaceutical Secondary Standard; Certified Reference Material
220. Medroxyprogesterone Acetate, United States Pharmacopeia (usp) Reference Standard
221. Medroxyprogesterone Acetate For Peak Identification, European Pharmacopoeia (ep) Reference Standard
222. Medroxyprogesterone Acetate For System Suitability, European Pharmacopoeia (ep) Reference Standard
223. Medroxyprogesterone Acetate, For Performance Test, European Pharmacopoeia (ep) Reference Standard
| Molecular Weight | 386.5 g/mol |
|---|---|
| Molecular Formula | C24H34O4 |
| XLogP3 | 4.1 |
| Hydrogen Bond Donor Count | 0 |
| Hydrogen Bond Acceptor Count | 4 |
| Rotatable Bond Count | 3 |
| Exact Mass | 386.24570956 g/mol |
| Monoisotopic Mass | 386.24570956 g/mol |
| Topological Polar Surface Area | 60.4 Ų |
| Heavy Atom Count | 28 |
| Formal Charge | 0 |
| Complexity | 767 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 7 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 1 |
| 1 of 6 | |
|---|---|
| Drug Name | DEPO-PROVERA |
| Active Ingredient | MEDROXYPROGESTERONE ACETATE |
| Company | PHARMACIA AND UPJOHN (Application Number: N012541); PHARMACIA AND UPJOHN (Application Number: N020246) |
| 2 of 6 | |
|---|---|
| Drug Name | DEPO-SUBQ PROVERA 104 |
| Active Ingredient | MEDROXYPROGESTERONE ACETATE |
| Company | PHARMACIA AND UPJOHN (Application Number: N021583. Patent: 6495534) |
| 3 of 6 | |
|---|---|
| Drug Name | MEDROXYPROGESTERONE ACETATE |
| Active Ingredient | MEDROXYPROGESTERONE ACETATE |
| Company | AMPHASTAR PHARMS INC (Application Number: A077235); AMPHASTAR PHARMS INC (Application Number: A077334); BARR (Application Number: A040159); TEVA PHARMS USA (Application Number: A076553) |
| 4 of 6 | |
|---|---|
| Drug Name | PROVERA |
| Active Ingredient | MEDROXYPROGESTERONE ACETATE |
| Company | PHARMACIA AND UPJOHN (Application Number: N011839) |
| 5 of 6 | |
|---|---|
| Drug Name | PREMPHASE 14/14 |
| Active Ingredient | ESTROGENS, CONJUGATED; MEDROXYPROGESTERONE ACETATE |
| Company | WYETH PHARMS INC (Application Number: N020527) |
| 6 of 6 | |
|---|---|
| Drug Name | PREMPRO |
| Active Ingredient | ESTROGENS, CONJUGATED; MEDROXYPROGESTERONE ACETATE |
| Company | WYETH PHARMS INC (Application Number: N020527) |
Medroxyprogesterone acetate (MPA) oral tablets are indicated to treat secondary amenorrhea, reduce the incidence of endometrial hyperplasia in postmenopausal women, and to treat abnormal uterine bleeding due to hormonal imbalance, not organic pathology. Oral tablets containing MPA and conjugated estrogens are indicated to prevent postmenopausal osteoporosis and to treat moderate to severe menopausal symptoms such as vasomotor symptoms, vulvar atrophy, and vaginal atrophy. Subcutaneous MPA is indicated to prevent pregnancy and manage pain associated with endometriosis. Intramuscular MPA is indicated to prevent pregnancy, and at higher concentrations for palliative treatment of endometrial or renal carcinoma.
FDA Label
Medroxyprogesterone acetate (MPA) inhibits gonadotropin production, reduces nuclear estrogen receptors and DNA synthesis in epithelial cells of the endometrium, and induces p53 dependant apoptosis in cancer cell lines. MPA oral tablets have a half life of 40-60 hours and other formulations can have half lives that are considerably longer, so the duration of action is long. The therapeutic window is wide as patients may take doses ranging from 5mg orally daily to 1000mg as a depo injection weekly. Long term use of MPA is associated with a reduction in bone density and patients who taking MPA during adolescence may have lower peak bone mass than untreated patients, which can also increase the risk of osteoporosis and fractures in the future.
Contraceptive Agents, Female
Chemical substances or agents with contraceptive activity in females. Use for female contraceptive agents in general or for which there is no specific heading. (See all compounds classified as Contraceptive Agents, Female.)
Contraceptive Agents, Male
Chemical substances or agents with contraceptive activity in males. Use for male contraceptive agents in general or for which there is no specific heading. (See all compounds classified as Contraceptive Agents, Male.)
Antineoplastic Agents, Hormonal
Antineoplastic agents that are used to treat hormone-sensitive tumors. Hormone-sensitive tumors may be hormone-dependent, hormone-responsive, or both. A hormone-dependent tumor regresses on removal of the hormonal stimulus, by surgery or pharmacological block. Hormone-responsive tumors may regress when pharmacologic amounts of hormones are administered regardless of whether previous signs of hormone sensitivity were observed. The major hormone-responsive cancers include carcinomas of the breast, prostate, and endometrium; lymphomas; and certain leukemias. (From AMA Drug Evaluations Annual 1994, p2079) (See all compounds classified as Antineoplastic Agents, Hormonal.)
Contraceptive Agents, Hormonal
Contraceptive agents that act on the ENDOCRINE SYSTEM. (See all compounds classified as Contraceptive Agents, Hormonal.)
Absorption
Absorption of oral medroxyprogesterone acetate (MPA) varies considerably between formulations. A 1000mg oral dose reaches an average Cmax of 145-315nmol/L while a 500mg oral dose reaches an average Cmax of 33-178nmol/L with a Tmax of 1-3 hours and a lag time of half an hour. The AUC of a 500mg oral dose of MPA was 543.4-1981.1nmol\*L/h depending on formulation. Intramuscular MPA reaches a Cmax of 4.691.52nmol/L with a Tmax of 4.752.09 days and an AUC of 81.5827.64days\*nmol/L. Subcutaneous MPA reaches a Cmax of 3.831.56nmol/L with a Tmax of 6.522.07 days and an AUC of 72.2638.73days\*nmol/L. However, the pharmacokinetics of MPA may also vary depending on injection site.
Route of Elimination
The majority of medroxyprogesterone acetate (MPA) is eliminated in the urine as glucuronide conjugates and a minority of sulphate conjugates. Glucuronide conjugates are also detected in the feces. Determining the exact ratio of metabolites and parent compound eliminated in the urine and feces is difficult as the metabolite profile in the urine is not significantly different and radio labelling studies are not readily available.
Volume of Distribution
The volume of distribution of medroxyprogesterone acetate is 203L.
Clearance
The mean clearance of medroxyprogesterone acetate (MPA) is 1668146L/day or 21.94.3L/kg/day. Due to the high inter patient variability in MPA pharmacokinetics, clearance has been reported to be 1600-4000L/day.
Medroxyprogesterone acetate undergoes beta hydroxylation to form the metabolites 6-beta (M-2), 2-beta (M-4), and 1-beta-hydroxymedroxyprogesterone acetate (M-3). M-2 and M-4 are further metabolized to 2-beta,6-beta-dihydroxymedroxyprogesterone (M-1). M-3 is further metabolized to 1,2-dehydromedroxyprogesterone acetate (M-5).
Medroxyprogesterone Acetate has known human metabolites that include M-2, M-3, and Medroxyprogesterone Acetate.
S73 | METXBIODB | Metabolite Reaction Database from BioTransformer | DOI:10.5281/zenodo.4056560
Oral medroxyprogesterone acetate (MPA) has an absorption half life of 15-30min and a biological half life of 40-60 hours. Intramuscular MPA has an absorption half life of 0.860.30 days and an elimination half life of 24.0321.74 days. Subcutaneous MPA has an absorption half life of 1.050.56 days and an elimination half life of 30.9015.11 days.
Medroxyprogesterone acetate (MPA) inhibits the production of gonadotropin, preventing follicular maturation and ovulation, which is responsible for its ability to prevent pregnancy. This action also thins the endometrium. MPA reduces nuclear estrogen receptors and DNA synthesis in epithelial cells of the endometrium. MPA can also induce p53 dependant apoptosis in certain cancer cell lines, and inhibit GABA-A receptors.
BUILDING BLOCK

