
1. 156-s
2. 2-(4-((2-oxocyclopentyl)methyl)phenyl)propionic Acid
3. 2-ocppp
4. Cs 600
5. Cs-600
6. Loxoprofen
7. Loxoprofen Alcohol
8. Loxoprofen Sodium Dihydrate
9. Loxoprofen Sodium, (r*,s*)-isomer
10. Sodium 2-(4-(2-oxocyclopentylmethyl)phenyl)propionate Dihydrate
11. Sodium Loxoprofen
1. 80382-23-6
2. Loxoprofen Sodium Salt
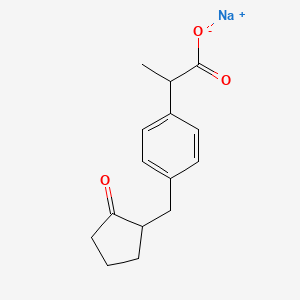
3. Sodium 2-(4-((2-oxocyclopentyl)methyl)phenyl)propanoate
4. Cs-600
5. Loxoprofen (sodium)
6. Sodium Loxoprofen
7. Lx-a
8. Sodium;2-[4-[(2-oxocyclopentyl)methyl]phenyl]propanoate
9. Loxoprofen Sodium Hydrate
10. Ndc2m7399s
11. Chebi:76198
12. Mfcd00941425
13. Chembl66552
14. Sodium 2-(4-(2-oxocyclopentylmethyl)phenyl)propionate Dihydrate
15. Alpha-methyl-4-((2-oxocyclopentyl)methyl)benzeneacetate Sodium Salt Dihydrate
16. Sodium 2-{4-[(2-oxocyclopentyl)methyl]phenyl}propanoate
17. Unii-ndc2m7399s
18. Lp-x
19. Mls001401445
20. Schembl383675
21. Sodium 2-[4-[(2-oxocyclopentyl)methyl]phenyl]propionate
22. Hy-b0578a
23. Amy8901
24. Cs-600g
25. Hms2051d12
26. Hms2233j14
27. Hms3370c11
28. Hms3393d12
29. Loxoprofen Sodium [who-dd]
30. Loxoprofen Sodium Salt [mi]
31. Akos025392177
32. Ccg-101024
33. Ccg-267132
34. Ks-5040
35. Loxoprofen Sodium Hydrate [jan]
36. Nc00274
37. Bl164648
38. Smr000469165
39. Cs-0013894
40. Ft-0630879
41. L0252
42. A839900
43. Q-201325
44. Q27145804
45. Sodium2-(4-((2-oxocyclopentyl)methyl)phenyl)propanoate
46. 2-{4-[(2-oxocyclopentyl)methyl]phenyl}propanoate Sodium Salt
47. 2-[4-[(2-oxocyclopentyl)methyl]phenyl]propionic Acid Sodium Salt
48. Sodium 2-(4-((2-oxocyclopentyl)methyl)(phenyl)propionate
49. Benzeneacetic Acid, Alpha-methyl-4-((2-oxocyclopentyl)methyl)-, Sodium Salt, Dihydrate
50. Benzeneacetic Acid, .alpha.-methyl-4-((2-oxocyclopentyl)methyl)-, Sodium Salt (1:1)
| Molecular Weight | 268.28 g/mol |
|---|---|
| Molecular Formula | C15H17NaO3 |
| Hydrogen Bond Donor Count | 0 |
| Hydrogen Bond Acceptor Count | 3 |
| Rotatable Bond Count | 4 |
| Exact Mass | 268.10753868 g/mol |
| Monoisotopic Mass | 268.10753868 g/mol |
| Topological Polar Surface Area | 57.2 Ų |
| Heavy Atom Count | 19 |
| Formal Charge | 0 |
| Complexity | 321 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 0 |
| Undefined Atom Stereocenter Count | 2 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 2 |
Anti-Inflammatory Agents, Non-Steroidal
Anti-inflammatory agents that are non-steroidal in nature. In addition to anti-inflammatory actions, they have analgesic, antipyretic, and platelet-inhibitory actions. They act by blocking the synthesis of prostaglandins by inhibiting cyclooxygenase, which converts arachidonic acid to cyclic endoperoxides, precursors of prostaglandins. Inhibition of prostaglandin synthesis accounts for their analgesic, antipyretic, and platelet-inhibitory actions; other mechanisms may contribute to their anti-inflammatory effects. (See all compounds classified as Anti-Inflammatory Agents, Non-Steroidal.)
