




1. 853029-57-9
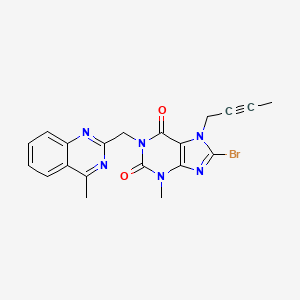
2. 8-bromo-7-(but-2-yn-1-yl)-3-methyl-1-((4-methylquinazolin-2-yl)methyl)-1h-purine-2,6(3h,7h)-dione
3. 1-[(4-methylquinazolin-2-yl)methyl]-3-methyl-7-(2-butyn-1-yl)-8-bromoxanthine
4. 8-bromo-7-(2-butyn-1-yl)-3,7-dihydro-3-methyl-1-[(4-methyl-2-quinazolinyl)methyl]-1h-purine-2,6-dione
5. 8-bromo-7-but-2-ynyl-3-methyl-1-[(4-methylquinazolin-2-yl)methyl]purine-2,6-dione
6. 8-bromo-7-(2-butyn-1-yl)-3,7-dihydro-3-methyl-1-((4-methyl-2-quinazolinyl)methyl)-1h-purine-2,6-dione
7. 8-bromo-7-(but-2-yn-1-yl)-3-methyl-1-[(4-methylquinazolin-2-yl)methyl]-3,7-dihydro-1h-purine-2,6-dione
8. 8-bromo-7-(2-butyn-1-yl)-3,7-dihydro-3-methyl-1-[(4-methyl-2-quinazolinyl)methyl]-1h-purine-2,6-dion
9. Schembl3919837
10. Dtxsid00647176
11. Bcp11833
12. Cs-m2881
13. Mfcd18642578
14. Zinc72190311
15. Akos016004953
16. Kg-0209
17. Ac-23922
18. Da-19419
19. Am20090703
20. Ft-0699074
21. A26902
22. F19248
23. J-519398
24. 1-[(4-methyl-quinazolin-2-yl)methyl]-3-methyl-7-(2-butyn-1-yl)-8-bromo Xanthine
25. 1-[(4-methyl-quinazolin-2-yl)methyl]-3-methyl-7-(2-butyn-1-yl)-8-bromoxanthine
26. 1-[(4-methyl-quinazolin-2yl) Methyl]-3-methyl-7-(2-butyn-1-yl)-8-bromo-xanthine
27. 1-[(4-methylquinazolin-2-yl)methyl]-3-methyl-7-(2-butin-1-yl)-8-bromoxanthine
28. 1h-purine-2,6-dione, 8-bromo-7-(2-butyn-1-yl)-3,7-dihydro-3-methyl-1-[(4-methyl-2-quinazolinyl)methyl]-
29. 8-bromo-7-(but-2-yn-1-yl)-3-methyl-1-((4-methylquin-azolin-2-yl)methyl)-1h-purine-2,6(3h,7h)-dione
30. 8-bromo-7-(but-2-yn-1-yl)-3-methyl-1-[(4-methylquinazolin-2-yl)methyl]-2,3,6,7-tetrahydro-1h-purine-2,6-dione
31. 8-bromo-7-(but-2-ynyl)-3-methyl-1-((4-methylquinazolin-2-yl)methyl)-1h-purine-2,6(3h,7h)-dione
32. 8-bromo-7-(but-2-ynyl)-3-methyl-1-(4-methyl-quinazolin-2-ylmethyl)-3,7-dihydro-purine-2,6-dione
| Molecular Weight | 453.3 g/mol |
|---|---|
| Molecular Formula | C20H17BrN6O2 |
| XLogP3 | 2.9 |
| Hydrogen Bond Donor Count | 0 |
| Hydrogen Bond Acceptor Count | 5 |
| Rotatable Bond Count | 3 |
| Exact Mass | 452.05964 g/mol |
| Monoisotopic Mass | 452.05964 g/mol |
| Topological Polar Surface Area | 84.2 Ų |
| Heavy Atom Count | 29 |
| Formal Charge | 0 |
| Complexity | 735 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 0 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 1 |


