

X



1. 874649-82-8
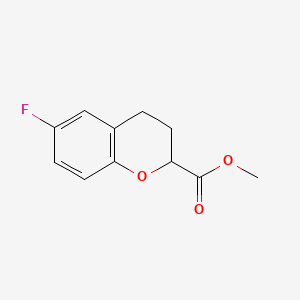
2. Methyl 6-fluorochromane-2-carboxylate
3. Methyl 6-fluoro-3,4-dihydro-2h-chromene-2-carboxylate
4. Rac-6-fluoro-3,4-dihydro-2h-1-benzopyran-2-carboxylic Acid Methyl Ester
5. Methyl 6-fluoro-3,4-dihydro-2h-1-benzopyran-2-carboxylate
6. Schembl2005546
7. Dtxsid00595128
8. Mfcd09701969
9. Akos015853416
10. Ds-2072
11. Ts-02990
12. 2-methylamino-1-phenylethanonehydrochloride
13. Cs-0001991
14. Ft-0658816
15. M2879
16. A842225
17. 2h-1-benzopyran-2-carboxylicacid,6-fluoro-3,4-dihydro-,methyl Ester
| Molecular Weight | 210.20 g/mol |
|---|---|
| Molecular Formula | C11H11FO3 |
| XLogP3 | 2.3 |
| Hydrogen Bond Donor Count | 0 |
| Hydrogen Bond Acceptor Count | 4 |
| Rotatable Bond Count | 2 |
| Exact Mass | g/mol |
| Monoisotopic Mass | g/mol |
| Topological Polar Surface Area | 35.5 |
| Heavy Atom Count | 15 |
| Formal Charge | 0 |
| Complexity | 244 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 0 |
| Undefined Atom Stereocenter Count | 1 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 1 |




