




1. 875781-41-2
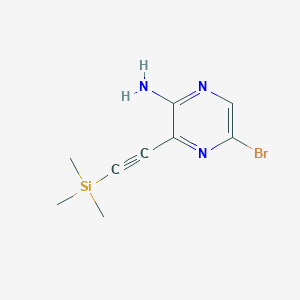
2. 5-bromo-3-[(trimethylsilyl)ethynyl]pyrazin-2-amine
3. 5-bromo-3-(2-(trimethylsilyl)ethynyl)pyrazin-2-amine
4. 5-bromo-3-(2-trimethylsilylethynyl)pyrazin-2-amine
5. Mfcd16660032
6. 5-bromo-3-trimethylsilanylethynylpyrazin-2-ylamine
7. C9h12brn3si
8. Schembl422711
9. Amy3323
10. Dtxsid80693833
11. Lqjgzefwbxjkji-uhfffaoysa-n
12. Cs-m3193
13. Akos015892473
14. Ds-14236
15. Sy011001
16. Ft-0716538
17. A850456
18. 5-bromo-3-trimethylsilanylethynyl-pyrazin-2-ylamine
19. J-516999
20. 5-bromo-3-((trimethylsilyl)ethynyl)pyrazine-2-amine
21. 5-bromo-3-(2-(trimethylsilyl)ethynyl)pyrazin-2-amine;5-bromo-3-[(trimethylsilyl)ethynyl]pyrazin-2-amine
| Molecular Weight | 270.20 g/mol |
|---|---|
| Molecular Formula | C9H12BrN3Si |
| Hydrogen Bond Donor Count | 1 |
| Hydrogen Bond Acceptor Count | 3 |
| Rotatable Bond Count | 2 |
| Exact Mass | g/mol |
| Monoisotopic Mass | g/mol |
| Topological Polar Surface Area | 51.8 |
| Heavy Atom Count | 14 |
| Formal Charge | 0 |
| Complexity | 262 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 0 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 1 |


