

X


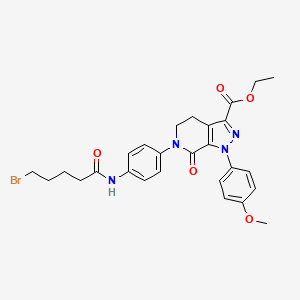

1. 881386-12-5
2. Ethyl 6-[4-(5-bromopentanoylamino)phenyl]-1-(4-methoxyphenyl)-7-oxo-4,5-dihydropyrazolo[3,4-c]pyridine-3-carboxylate
3. Schembl15174803
4. Zinc100469416
| Molecular Weight | 569.4 g/mol |
|---|---|
| Molecular Formula | C27H29BrN4O5 |
| XLogP3 | 4.4 |
| Hydrogen Bond Donor Count | 1 |
| Hydrogen Bond Acceptor Count | 6 |
| Rotatable Bond Count | 11 |
| Exact Mass | g/mol |
| Monoisotopic Mass | g/mol |
| Topological Polar Surface Area | 103 |
| Heavy Atom Count | 37 |
| Formal Charge | 0 |
| Complexity | 785 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 0 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 1 |







