




1. 898543-06-1
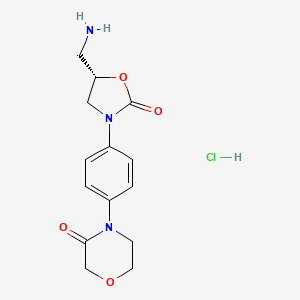
2. (s)-4-(4-(5-(aminomethyl)-2-oxooxazolidin-3-yl)phenyl)morpholin-3-one Hcl
3. 4-[4-[(5s)-5-(aminomethyl)-2-oxo-1,3-oxazolidin-3-yl]phenyl]morpholin-3-one Hydrochloride
4. 4-[4-[(5s)-5-(aminomethyl)-2-oxo-1,3-oxazolidin-3-yl]phenyl]morpholin-3-one;hydrochloride
5. 4-[4-[(5s)-5-(aminomethyl)-2-oxo-3-oxazolidinyl]phenyl]-3-morpholinone Hydrochloride
6. 4-{4-[(5s)-5-(aminomethyl)-2-oxo-1,3-oxazolidin-3-yl]phenyl}morpholin-3-one Hydrochloride
7. (s)-4-[4-[5-(aminomethyl)-2-oxo-3-oxazolidinyl]phenyl]morpholin-3-one Hydrochloride
8. 4-(4-((5s)-5-(aminomethyl)-2-oxo-1,3-oxazolidin-3-yl)phenyl)morpholin-3-one Hydrochloride
9. 4-(4-((5s)-5-(aminomethyl)-2-oxo-3-oxazolidinyl)phenyl)-3-morpholinone Hydrochloride
10. 4-[4-[(5s)-5-(aminomethyl)-2-oxooxazolidin-3-yl]phenyl]morpholin-3-one Hydrochloride
11. Ec 618-311-0
12. R5yc7w3x3c
13. Schembl792570
14. Dtxsid20581225
15. Cs-m1864
16. Mfcd20133901
17. Akos016001386
18. R-(-)-methamphetamine-d3 Hydrochloride
19. 1329647-30-4
20. 3-morpholinone, 4-[4-[(5s)-5-(aminomethyl)-2-oxo-3-oxazolidinyl]phenyl]-, Hydrochloride (1:1)
21. Ac-25295
22. As-19754
23. Da-18285
24. Am20090739
25. J-513406
26. 4-{4-[(5s)-5-aminomethyl)-2-oxo-1,3-oxazolidin-3-yl}morpholin-3-one
27. S)-4-(4-(5-(aminomethyl)-2-oxooxazolidin-3-yl)phenyl)morpholin-3-one.hcl
28. (s)-4-(4-(5-(aminomethyl)-2-oxooxazolidin-3-yl)phenyl)morpholin-3-onehydrochloride
29. (s)-4-{4-[5-(aminomethyl)-2-oxo-1,3-oxazolidin-3-yl]phenyl}morpholin-3-one Hydrochloride
30. 4-((s)-5-aminomethyl-2-oxo-oxazolidin-3-yl)-phenyl-morpholin-3-one Hydrochloride
31. 4-(4-(5-(aminomethyl)-2-oxooxazolidin-3-yl)phenyl)morpholin-3-one Hydrochloride
32. 4-[4-[(5s)-5-(aminomethyl)-2-oxo-3-oxazolidinyl]phenyl]-3-morpholinone Hydrochloride Salt
33. 4-{4-[(5s)-5-(amino Methyl)-2-oxo-1,3-oxazolidin-3-yl]phenyl}morpholin-3-one Hydrochloride
34. 4-{4-[(5s)-5-(aminomethyl)-2-oxo-1, 3-oxazolidin-3-yl] Phenyl} Morpholin-3-one Hydrochloride
35. 4-{4-[(5s)-5-(aminomethyl)-2-oxo-1,3-oxazolidin-3-yl]phenyl}morpholin-3-one--hydrogen Chloride (1/1)
36. 4-{4-[(5s)-5-aminomethyl-2-oxo-1,3-oxazolidine-3-yl]-phenyl}-morpholine-3-one Hydrochloride
| Molecular Weight | 327.76 g/mol |
|---|---|
| Molecular Formula | C14H18ClN3O4 |
| Hydrogen Bond Donor Count | 2 |
| Hydrogen Bond Acceptor Count | 5 |
| Rotatable Bond Count | 3 |
| Exact Mass | 327.0985838 g/mol |
| Monoisotopic Mass | 327.0985838 g/mol |
| Topological Polar Surface Area | 85.1 Ų |
| Heavy Atom Count | 22 |
| Formal Charge | 0 |
| Complexity | 409 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 1 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 2 |





