




1. 917389-21-0
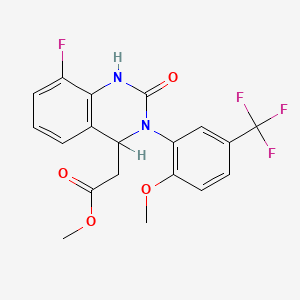
2. Methyl 2-(8-fluoro-3-(2-methoxy-5-(trifluoromethyl)phenyl)-2-oxo-1,2,3,4-tetrahydroquinazolin-4-yl)acetate
3. Schembl8746748
4. Cs-m2410
5. Akos037650396
6. Cs-14745
7. C12718
8. 4-quinazolineacetic Acid,8-fluoro-1,2,3,4-tetrahydro-3-[2-methoxy-5-(trifluoromethyl)phenyl]-2-oxo-,methyl Ester
9. Methyl {8-fluoro-3-[2-methoxy-5-(trifluoromethyl)phenyl]-2-oxo-1,2,3,4-tetrahydroquinazolin-4-yl}acetate
10. Methyl 2-[8-fluoro-3-[2-methoxy-5-(trifluoromethyl)phenyl]-2-oxo-1,4-dihydroquinazolin-4-yl]acetate
11. Methyl{8-fluoro-3-[2-methoxy-5-(trifluoromethyl)phenyl]-2-oxo-1,2,3,4-tetrahydroquinazolin-4-yl}acetate
12. Methyl2-(8-fluoro-3-(2-methoxy-5-(trifluoromethyl)phenyl)-2-oxo-1,2,3,4-tetrahydroquinazolin-4-yl)acetate
| Molecular Weight | 412.3 g/mol |
|---|---|
| Molecular Formula | C19H16F4N2O4 |
| XLogP3 | 3.1 |
| Hydrogen Bond Donor Count | 1 |
| Hydrogen Bond Acceptor Count | 8 |
| Rotatable Bond Count | 5 |
| Exact Mass | 412.10461964 g/mol |
| Monoisotopic Mass | 412.10461964 g/mol |
| Topological Polar Surface Area | 67.9 Ų |
| Heavy Atom Count | 29 |
| Formal Charge | 0 |
| Complexity | 618 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 0 |
| Undefined Atom Stereocenter Count | 1 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 1 |


