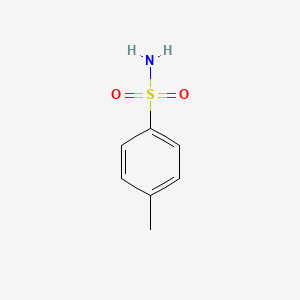

1. 4-methylbenzenesulfonamide
2. 4-toluenesulfonamide
3. 4-toluenesulfonamide Hydrochloride
4. 4-toluenesulfonamide, 6-(14)c-labeled
5. 4-toluenesulfonamide, Mercury (+2) Salt (1:1)
6. 4-toluenesulfonamide, Mercury (+2) Salt (2:1)
7. 4-toluenesulfonamide, Monopotassium Salt
8. 4-toluenesulfonamide, Monosilver Salt
9. 4-toluenesulfonamide, Monosodium Salt
10. 4-toluenesulfonylamide
11. Para-toluenesulfonamide
12. Para-toluenesulphonamide
1. 4-methylbenzenesulfonamide
2. 70-55-3
3. 4-toluenesulfonamide
4. P-tosylamide
5. Tosylamide
6. Toluene-4-sulfonamide
7. Benzenesulfonamide, 4-methyl-
8. Para-toluenesulfonamide
9. P-tolylsulfonamide
10. P-methylbenzenesulfonamide
11. P-toluenesulfonylamide
12. 4-methylbenzene-1-sulfonamide
13. Toluene-4-sulphonamide
14. Tolylsulfonamide
15. P-toluenesulfamide
16. Toluene-p-sulphonamide
17. Tosylamine
18. Toluenesulfonamide, P-
19. Nsc-9908
20. 4-methylbenzenesulphonamide
21. Chembl574
22. Chebi:34435
23. I8266ri90m
24. Dsstox_cid_9105
25. Dsstox_rid_78668
26. Dsstox_gsid_29105
27. 4-toluenesulfanamide
28. Cas-70-55-3
29. 4-toluenesulfonic Acid, Amide
30. Hsdb 5203
31. Nsc 9908
32. Einecs 200-741-1
33. Mfcd00011692
34. Brn 0472689
35. Unii-i8266ri90m
36. Ai3-19503
37. 4-tolylsulfonamide
38. P-toluensulfonamide
39. 4-tolylsulphonamide
40. 4j8
41. P-toluenesulphonamide
42. 4-toluenesulphonamide
43. P-toluene Sulfonamide
44. P-toluene-sulfonamide
45. Pasam
46. Toluene-p-sulfonamide
47. P-toluene Sulphonamide
48. N-p-tolylsulfonylamine
49. Paratoulene Sulfonamide
50. P-toluenesulfonic Amide
51. Para-toluen Sulphonamide
52. Para Toluene Sulfonamide
53. Para-toluene Sulphonamide
54. 4-methylphenylsulphonamide
55. 4-methylphenyl Sulfonamide
56. P-toluenesulfonamide,(s)
57. 4-methyl Benzenesulfonamide
58. 4-methyl-benzenesulfonamide
59. Wln: Zswr D1
60. 4-methylphenylsulphonylamine
61. Ec 200-741-1
62. P-methyl-benzene Sulfonamide
63. Schembl7370
64. 4-11-00-00376 (beilstein Handbook Reference)
65. Mls001065595
66. Bidd:er0609
67. Dtxsid8029105
68. Bdbm10859
69. Nsc9908
70. Hms3039b12
71. Zinc388056
72. P-toluenesulfonamide [hsdb]
73. Amy37180
74. Tox21_201594
75. Tox21_303506
76. Aromatic/heteroaromatic Sulfonamide 4
77. Stk416410
78. P-toluenesulfonamide [usp-rs]
79. Akos000149655
80. Fs-3585
81. Ncgc00091435-01
82. Ncgc00091435-02
83. Ncgc00091435-03
84. Ncgc00257435-01
85. Ncgc00259143-01
86. Bp-12584
87. Nci60_042220
88. P-toluenesulfonamide, Reagent Grade, 98%
89. Smr000568494
90. Bb 0258885
91. Cs-0012133
92. Ft-0646496
93. T0281
94. Tolbutamide Impurity A [ep Impurity]
95. P-toluenesulfonamide, Reagentplus(r), >=99%
96. Ae-562/40173366
97. P-toluenesulfonamide, Vetec(tm) Reagent Grade, 98%
98. W-104550
99. W-109664
100. Q23013959
101. Z45415564
102. F0722-8812
103. P-toluenesulfonamide, United States Pharmacopeia (usp) Reference Standard
104. 12552-95-3
| Molecular Weight | 171.22 g/mol |
|---|---|
| Molecular Formula | C7H9NO2S |
| XLogP3 | 0.8 |
| Hydrogen Bond Donor Count | 1 |
| Hydrogen Bond Acceptor Count | 3 |
| Rotatable Bond Count | 1 |
| Exact Mass | 171.03539970 g/mol |
| Monoisotopic Mass | 171.03539970 g/mol |
| Topological Polar Surface Area | 68.5 Ų |
| Heavy Atom Count | 11 |
| Formal Charge | 0 |
| Complexity | 209 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 0 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 1 |
/CLINICAL TRIALS/ ClinicalTrials.gov is a registry and results database of publicly and privately supported clinical studies of human participants conducted around the world. The Web site is maintained by the National Library of Medicine (NLM) and the National Institutes of Health (NIH). Each ClinicalTrials.gov record presents summary information about a study protocol and includes the following: Disease or condition; Intervention (for example, the medical product, behavior, or procedure being studied); Title, description, and design of the study; Requirements for participation (eligibility criteria); Locations where the study is being conducted; Contact information for the study locations; and Links to relevant information on other health Web sites, such as NLM's MedlinePlus for patient health information and PubMed for citations and abstracts for scholarly articles in the field of medicine. Para-toluenesulfonamide is included in the database.
NIH/NLM; ClinicalTrials.Gov. Available from, as of April 24, 2018: https://clinicaltrials.gov/
/EXPL THER/ Severe malignant airway obstruction (SMAO) is a life-threatening form of non-small cell lung carcinoma (NSCLC). /The purpose of this study was/ to determine the efficacy and safety of para-toluenesulfonamide (PTS) intratumoral injection in NSCLC-SMAO. Ninety patients with NSCLC-SAO received repeated courses of PTS intratumoral injection until tumor sizes had reduced by 50% or greater. Primary endpoint was objective alleviation rate, assessed by chest computed tomography (CT) and bronchoscopy, at day 7 and 30 following final dosing. Secondary endpoints included airway obstruction, spirometry, quality-of-life and survival time. In full-analysis set (N=88), using RECIST criteria, PTS treatment resulted in a significant objective alleviation rate [chest CT: 59.1% (95%CI: 48.1%-69.5%), bronchoscopy: 48.9% (95%CI: 38.1%-59.8%) at day 7; chest CT: 43.2% (95%CI: 32.7%-54.2%), bronchoscopy: 29.6% (95%CI: 20.3%-40.2%) at day 30]. There was a remarkable increase in FVC (mean difference: 0.35 liters, 95%CI: 0.16-0.53 liters), FEV1 (mean difference: 0.27 liters, 95%CI: 0.07-0.48 liters), Baseline Dyspnea Index (mean difference: 64.8%, 95%CI: 53.9-74.7%) and Functional Assessment of Cancer Therapy-Lung Cancer Subscale (mean difference: 6.9, 95%CI: 3.8-9.9) at day 7 post-treatment. We noted significantly reduced prevalence of atelectasis (by 42.9%) and Eastern Cooperative Oncology Group physical performance scale (mean difference: 7.2, 95%CI: 3.9-10.5). Median survival time was 394 days in full-analysis set and 460 days in per-protocol set. Adverse events were reported in 64.0% of subjects. Seven severe adverse events (7.9%) were reported, of which three led to death (drug-related in one case). PTS intratumoral injection is effective and well tolerated for palliative therapy of NSCLC-SMAO.
PMID:27393505 Li SY et al; Lung Cancer 98: 43-50 (2016)
/EXPL THER/ /The purpose was/ to study the effect of percutaneous para-toluenesulfonamide (PTS) injection on transplanted hepatocarcinoma in nude mice. Sixty nude mice with subcutaneous transplanted hepatocarcinoma were randomized into 6 groups, namely PTS, chemotherapy, radiotherapy, PTS+chemotherapy, PTS+radiotherapy and control groups. PTS were injected into the tumor in the nude mouse models as indicated, and the tumor growth rate and survival time of the mice were recorded. All the treatments resulted in effective arrest of the tumor growth, but the effects of PTS+chemotherapy and PTS+radiotherapy were more obvious. No significant difference in the survival time of the mice were noted between the groups. PTS+chemotherapy and PTS+radiotherapy are safe and reliable, and produces better effects than either radiotherapy or chemotherapy alone.
PMID:19460736 Meng H et al; Nan Fang Yi Ke Da Xue Xue Bao 29 (5): 1024-5 (2009)
/EXPL THER/ Para-toluenesulfonamide (PTS) has been implicated with anticancer effects against a variety of tumors. In the present study, we investigated the inhibitory effects of PTS on tongue squamous cell carcinoma (Tca-8113) and explored the lysosomal and mitochondrial changes after PTS treatment in vitro. High-performance liquid chromatography showed that PTS selectively accumulated in Tca-8113 cells with a relatively low concentration in normal fibroblasts. Next, the effects of PTS on cell viability, invasion, and cell death were determined. PTS significantly inhibited Tca-8113 cells' viability and invasive ability with increased cancer cell death. Flow cytometric analysis and the lactate dehydrogenase release assay showed that PTS induced cancer cell death by activating apoptosis and necrosis simultaneously. Morphological changes, such as cellular shrinkage, nuclear condensation as well as formation of apoptotic body and secondary lysosomes, were observed, indicating that PTS might induce cell death through disturbing lysosomal stability. Lysosomal integrity assay and western blot showed that PTS increased lysosomal membrane permeabilization associated with activation of lysosomal cathepsin B. Finally, PTS was shown to inhibit ATP biosynthesis and induce the release of mitochondrial cytochrome c. Therefore, our findings provide a novel insight into the use of PTS in cancer therapy.
PMID:26302210 Full text: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4588602 Liu Z et al; Anticancer Drugs 26 (10): 1026-33 (2015)
/EXPL THER/ Hepatocellular carcinoma (HCC) is difficult to eradicate due to its resilient nature. Portal vein is often involved in tumors of large size, which exclude the patient from surgical resection and local ablative therapy, such as percutaneous ethanol injection (PEI) and radiofrequency ablation (RFA) because they were considered neither effective nor safe. Currently, there is almost no effective treatment for HCC of such condition. As a unique antitumor agent in form of lipophilic fluid for local injection, para-toluenesulfonamide (PTS) produces mild side effects while necrotizing the tumor tissues quickly and efficiently. Being largely different from both PEI and RFA therapies, PTS can disseminate itself in tumors more easily than other caustic agents, such as alcohol. So PTS may offer additional benefit to HCCs with vascular involvement. We herein describe a 70-year-old HCC patient who was treated with the combination of PTS injection and transcatheter arterial chemoembolization, resulting in a significantly improved clinical prognosis.
PMID:23239926 Full text: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3520177 He Q et al; World J Gastroenterol 18 (46): 6861-4 (2012)
Urinary excretion of administration p-toluenesulfonamide in rats was approx 80% with half of original compound being oxidized to p-sulfamoylbenzoic acid. More than 90% of /the p-sulfamoylbenzoic acid metabolite/ was excreted unchanged, but urine to feces ratios varied considerably among individual animals.
Minegishi KI et al; Chem Pharm Bull (Tokyo) 20 (7): 1351-6 (1972)
After oral administration of (14)C-labeled compound to rats the label was rapidly eliminated largely in urine (66-89% of dose), with little in feces (2-8% of dose). (14)C in feces was 4-sulfamoylbenzoic acid, which probably originated in tissues.
PMID:654313 Ball LM et al; Xenobiotica 8 (3): 183-90 (1978)
/The purpose of this study was/ to study the in vivo and in vitro metabolism and the effect of para-toluene-sulfonamide (PTS) on cytochrome P450 enzymes (CYP450). Total CYP450 and microsome protein content were determined after iv pretreatment of rats with PTS. CYP-specific substrates were incubated with rat liver microsomes. Specific CYP isoform activities were determined by using HPLC. CYP chemical inhibitors added to the incubation mixture were used to investigate the principal CYP isoforms involved in PTS metabolism. The effect of PTS on CYP isoforms was investigated by incubating PTS with specific substrates. The groups treated with 33 and 99 mg/kg per d PTS, respectively, had a total CYP content of 0.66+/-0.17 and 0.60+/-0.12 nmol/mg. The K(m) and V(max) were 92.2 umol/L and 0.0137 nmol/min per mg protein. CYP2C7, CYP2D1 and CYP3A2 might contribute to PTS metabolism in the rat liver. The inhibitory effects of sulfaphenazole and ketoconazole on PTS metabolism were shown to have a mixed mechanism, whereas PTS metabolism was inhibited noncompetitively by quinidine. PTS had little effect on the activities of the selected CYP isoforms. Generally speaking, it is relatively safe for PTS to be co-administered with other drugs. However, care should be taken when administering PTS with CYP inhibitors and the substrates of CYP2C, CYP2D and CYP3A.
PMID:16626521 Zhou JQ et al; Acta Pharmacol Sin 27 (5): 635-40 (2006)
Aim of this study was to investigate liver metabolism of with regard to para toluene-sulfonamide (PTS), CYP isoforms, P-glycoprotein (P-gp), and drug interactions. Known substrates, inducers and inhibitors of CYP and inhibitor of P-gp were employed and metabolites were determined with HPLC. Male Wistar rats were pretreated with ip phenobarbital (PB), ketoconazole (Ket), or verapamil (Ver) for 3 days and in situ liver perfusion of PTS was conducted in a recirculation system. Rats were also pretreated with ip PTS (33 mg/kg/day or PTS 99 mg/kg/day) for 4 days before liver perfusions with dextromethorphan (Dex) and phenacetin (Phe) preparations were conducted. Microsome incubation was used to investigate PTS effect on five CYP isoforms and PTS-drug interactions probability with phyllotoxin and 5-fluorouracil (5-FU) in vitro. PTS at 60 min perfusates had areas of 61.4% and 133.6% of the blank control in PB group and Ket group, respectively. The result that PTS metabolism was enhanced by PB and inhibited by Ket treatments suggested liver CYP was attributed to PTS metabolism. PTS mg/kg/day pretreatment slowed down the metabolism of Dex and Phe while in vitro incubations did not show a PTS (0-160 umol/L) effect on CYP activities. PTS metabolite formation when co-incubated with phyllotoxin was 50.7% of the negative control. The potent inhibitory ability of phyllotoxin to PTS requires further clinical investigation regarding in concomitant administration.
PMID:17069428 Zhou JQ et al; Pharmazie 61 (10): 869-73 (2006)
Fifteen adult rainbow trout were exposed to water solution of (14)C-tosylchloramide sodium (purity 93.7%, specific activity 1.2 ugCi/uM) at a concentration of 20 mg/L (twice the proposed treatment concentration) for a period of 1 hour and then transferred to fresh water. The temperature of the well water was 11 to 13 C. ... tosylchloramide sodium was rapidly reduced to the primary metabolite para-toluenesulfonamide, but the levels were not quantified.
European Agency for the Evaluation of Medicinal Products; Committee for Veterinary Medicinal Products; Tosylchloramide Sodium Summary Report (February 1999) EMEA/MRL/570/99-FINAL. Available from, as of November 8, 2017: https://www.ema.europa.eu/docs/en_GB/document_library/Maximum_Residue_Limits_-_Report/2009/11/WC500015637.pdf
...50% of admin ortho- and para-toluenesulfonamides excreted in urine had been metabolized to ortho- and para-sulfamoylbenzoic acids, respectively.
IARC. Monographs on the Evaluation of the Carcinogenic Risk of Chemicals to Humans. Geneva: World Health Organization, International Agency for Research on Cancer, 1972-PRESENT. (Multivolume work). Available at: https://monographs.iarc.fr/ENG/Classification/index.php, p. V73 573 (1999)
For more Metabolism/Metabolites (Complete) data for p-Toluenesulfonamide (6 total), please visit the HSDB record page.
Fingerlings and juvenile trout were exposed to 20 mg/L (twice the therapeutic concentration) of ring UL-14C-tosylchloramide sodium (purity 93.7%, specific activity 1.2 uCi/uM) for up to 1 hr and then transferred to fresh water for recovery to assess tissue accumulation and distribution of resulting residues. The temperature of the well water was 11.6 to 12.2 C. The estimated half-life of para-toluenesulfonamide equivalents in fingerlings was 27.3 hours whereas determined by HPLC the half-life of para-toluenesulfonamide residues in whole-body homogenates was 36.3 hours. The estimated half-life of residues in juvenile fish was 32.6 hours, based on radiometric data, while determined by HPLC the half-life for para-toluenesulfonamide residues in whole body samples was 40.3 hours.
European Agency for the Evaluation of Medicinal Products; Committee for Veterinary Medicinal Products; Tosylchloramide Sodium Summary Report (February 1999) EMEA/MRL/570/99-FINAL. Available from, as of November 8, 2017: https://www.ema.europa.eu/docs/en_GB/document_library/Maximum_Residue_Limits_-_Report/2009/11/WC500015637.pdf
BUILDING BLOCK

CAS Number : 70-55-3
End Use API :
End Use API : Tolazamide
About the Company : Suanfarma founded in 1993, is a B2B life science partner committed to health & innovation by developing, manufacturing, & distributing high-quality APIs for the pharmaceutical industry ...
