

X



1. 1006376-62-0
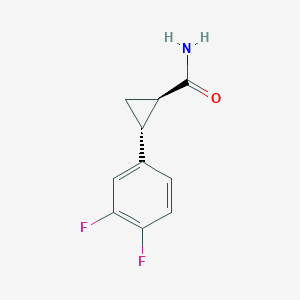
2. (1r,2r)-2-(3,4-difluorophenyl)cyclopropane-1-carboxamide
3. (1r,2r)-2-(3,4-difluorophenyl)cyclopropane Carboxamide
4. 1006614-51-2
5. Rel-(1r,2r)-2-(3,4-difluorophenyl)cyclopropane-1-carboxamide
6. Schembl2397845
7. Amy3282
8. Dtxsid30657804
9. Mfcd22380631
10. Ac-32549
11. Cs-15280
12. Cs-0009935
13. I11942
| Molecular Weight | 197.18 g/mol |
|---|---|
| Molecular Formula | C10H9F2NO |
| XLogP3 | 1.2 |
| Hydrogen Bond Donor Count | 1 |
| Hydrogen Bond Acceptor Count | 3 |
| Rotatable Bond Count | 2 |
| Exact Mass | g/mol |
| Monoisotopic Mass | g/mol |
| Topological Polar Surface Area | 43.1 |
| Heavy Atom Count | 14 |
| Formal Charge | 0 |
| Complexity | 246 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 2 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 1 |





