


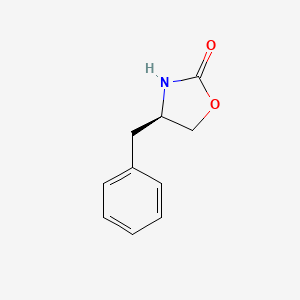

1. 102029-44-7
2. (r)-(+)-4-benzyl-2-oxazolidinone
3. (r)-4-benzyloxazolidin-2-one
4. (4r)-4-benzyl-1,3-oxazolidin-2-one
5. 2-oxazolidinone, 4-(phenylmethyl)-, (4r)-
6. R-4-benzyl-2-oxazolidinone
7. (r)-(-)-4-benzyl-2-oxazolidinone
8. (r)-4-benzyl-oxazolidin-2-one
9. R-(-)-4-benzyl-2-oxazolidinone
10. Mfcd00010846
11. 4-benzyl-1,3-oxazolidin-2-one #
12. R-(+)-4-benzyl-2-oxazolidinone
13. Mls001242802
14. 4(r)-benzyl-2-oxazolidinone
15. 4alpha-benzyl-2-oxazolidinone
16. Schembl137215
17. 4-(r)-benzyl-2-oxazolidinone
18. 4-(r)-benzyloxazolidin-2-one
19. (r)-4-benzyl-oxazolidin-2-on
20. (r)-4-benzyloxazolidine-2-one
21. Chembl1366601
22. (r)-4-benzyl Oxazolidin-2-one
23. Dtxsid60370594
24. (r) -4-benzyl-oxazolidin-2-one
25. Hms2205p17
26. (r)-(+)-4-benzyl-2-oxazolidone
27. (r)-(+)4-benzyl-2-oxazolidinone
28. Act01862
29. Bcp22798
30. Zinc4284392
31. Akos005145805
32. Akos015839026
33. (4r)-(+)-4-benzyl-2-oxazolidinone
34. (r)-4-(phenylmethyl)-2-oxazolidinone
35. (r)-4-benzyl-2-oxazolidinone, 99%
36. Ac-1396
37. Bcp9000029
38. Cs-w007664
39. Fs-2114
40. (r)-(+/-)-4-benzyl-2-oxazolidinone
41. Bp-12372
42. Ds-14383
43. Smr000841429
44. Db-005017
45. A1783
46. Am20060480
47. (4r)-4-(phenylmethyl)-1,3-oxazolidin-2-one
48. 29b447
49. A1-00440
50. Q-102351
| Molecular Weight | 177.20 g/mol |
|---|---|
| Molecular Formula | C10H11NO2 |
| XLogP3 | 1.7 |
| Hydrogen Bond Donor Count | 1 |
| Hydrogen Bond Acceptor Count | 2 |
| Rotatable Bond Count | 2 |
| Exact Mass | 177.078978594 g/mol |
| Monoisotopic Mass | 177.078978594 g/mol |
| Topological Polar Surface Area | 38.3 Ų |
| Heavy Atom Count | 13 |
| Formal Charge | 0 |
| Complexity | 187 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 1 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 1 |
 IKF/Pharmasynthese have been with fine chemicals market and APIs performance for more than 40 years.
IKF/Pharmasynthese have been with fine chemicals market and APIs performance for more than 40 years. 



