
1. Schembl2549414
2. Dtxsid20862647
3. Bcp06938
4. Mfcd16621007
5. Nsc358284
6. 1-(
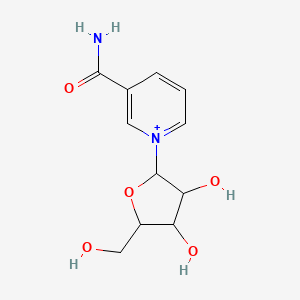
7. A-d-ribofuranosyl)-nicotinamide
8. Bcp06938-1
9. Nsc-358284
10. Sy226268
11. 3-carbamoyl-1-pentofuranosylpyridin-1-ium
12. Ns00122559
13. Nicotinamide Ribose; Nicotinamide-beta-riboside; Srt647; Srt-647; Srt 647
14. 1-((2r,3r,4s,5r)-3,4-dihydroxy-5-(hydroxymethyl)-tetrahydrofuran-2-yl)-1,2-dihydropyridine-3-carboxamide;nicotinamide Ribose; Nicotinamide Riboside; Ribonucleoside
| Molecular Weight | 255.25 g/mol |
|---|---|
| Molecular Formula | C11H15N2O5+ |
| XLogP3 | -1.8 |
| Hydrogen Bond Donor Count | 4 |
| Hydrogen Bond Acceptor Count | 5 |
| Rotatable Bond Count | 3 |
| Exact Mass | g/mol |
| Monoisotopic Mass | g/mol |
| Topological Polar Surface Area | 117 |
| Heavy Atom Count | 18 |
| Formal Charge | 1 |
| Complexity | 314 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 0 |
| Undefined Atom Stereocenter Count | 4 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 1 |
